Question
Question: Write the Lewis dot structure of \( CO \) molecule....
Write the Lewis dot structure of CO molecule.
Solution
Hint : Lewis dot structure deals with bond formation by representation of electrons around atoms. Count the number valence electrons of each atom then form the bond by completing the octet of the least electronegative atom.
Complete Step By Step Answer:
This is a question related to geometry of molecules. Here we are dealing with Lewis dot structure representation of molecules. So first let us see what Lewis dot structure is.
Lewis dot structure is named after American chemist Gilbert Newton Lewis. It is also called electron dot structure. It deals with representation of molecules that describes chemical bonding between atoms in a molecule. It also represents the total number of lone pairs in the atoms of the molecule.
Now let us see how a Lewis dot structure is drawn.
First the least electronegative atom is chosen in the molecule to be the central atom.
Remaining atoms are placed surrounding the central atom.
Now the valence electrons of each atom are represented as dots around the atom.
Now single bonds are used to connect atoms.
The remaining atoms form lone pairs.
Now let us see the Lewis dot structure of carbon monoxide. CO contains carbon and oxygen atoms. Carbon has four valence electrons while oxygen has six. The least electronegative among the two is carbon so carbon will be considered as the central atom although there are only two atoms in the molecule. Carbon needs four electrons for stable electronic configuration while oxygen needs two. Oxygen forms two single bonds by sharing two electrons and by sharing a lone pair to complete carbon’s octet. So oxygen and carbon are left with one lone pair and a triple bond is formed between the atoms. The same is shown below,
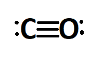
Note :
Lewis dot structure is mainly used for representation for covalent and coordinate compounds. It is helpful in determining lone pairs of a molecule. It doesn't matter where you place the dots around an atom.
