Question
Question: Write the IUPAC name of the given compound 
Solution
Before finding the IUPAC name, we first need to identify the functional groups present in the compound. After identifying all of the functional groups, we need to classify them as primary and secondary and then go for the nomenclature. Also we need to find the longest carbon chain to write the root word.
Complete step by step solution:
-To find the name, first we must find the functional groups present in the given structure. Here we see that there is presence of double bond(=), an alcohol group(-OH) and a methyl group which may or may not be included in the parent chain.
-The general method of writing the IUPAC name is
Secondary prefix+primary prefix+root word+primary suffix+seconday suffix.
The word root depends on the number of carbon atoms present in the longest chain. Eg:
| No. of C atoms in chain | Word root | No. of C atoms in chain | Word root |
|---|---|---|---|
| 1 | Meth | 8 | Oct |
| 2 | Eth | 9 | Non |
| 3 | Prop | 10 | Dec |
| 4 | But | 11 | Undec |
| 5 | pent | 20 | Icos |
| 6 | Hex | 30 | Triacont |
| 7 | Hept | 100 | cent |
-Primary suffix includes the saturation of compounds. Single bond is alkane, double bond alkene and triple bond alkyne.
-Secondary prefix and suffix is added to indicate the functional groups. They have priorities assigned to them. Higher priority functional group is written as a suffix and all functional groups with lower priority are written as a prefix. Some of the groups can be shown as
| CLASS | NAME | SUFFIX | PREFIX |
|---|---|---|---|
| R-COOH | Alkanoic acid | Oic acid | Carboxy |
| R−SO3H | Alkane sulfonic acid | Sulfonic acid | Sulfo |
| R-(CO)-O-(CO)-R | Alkanoic anhydride | Oic anhydride | ----- |
| R-COOR | Alkyl alkanoate | oate | Alkoxy carbonyl |
| R-(CO)-X | Alkanoyl halide | Oyl-halide | Halo Carbonyl |
| R−(CO)−NH2 | Alkanamide | Amide | Carbamoyl |
| R-CN | Alkanenitrile | Nitrile | Cyano |
| R-CHO | Alkanal | -al | Oxo |
| R-CO-R | Alkanone | -one | oxo |
| R-OH | Alkanol | -ol | Hydroxy |
| R-SH | Alkanethiol | -thiol | mercapto |
| R−NH2 | alkanamide | -amine | amino |
-Numbering is done such that double/triple bonds are given the highest location. Here, no multiple bond or primary functional group is present. So numbering has to start until the longest carbon chain is achieved along with the minimum sum of the locants. It can be shown as
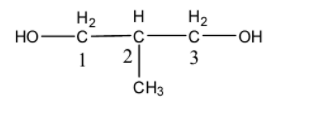
-So we see that there are 2 –OH groups at 1 and 3 positions and 1 Me group at position 2. The longest carbon chain is 3 and the root word is hence prop. Diol is written due to presence of 2 –OH groups and methyl is added due to the Me functional group.
Therefore the IUPAC name of the compound is 2-methylpropan-1,3-diol
Note: Always follow all the steps of naming a compound in proper sequence. The sequence is mandatory in the naming. The priorities of different functional groups need to be memorised as it plays an important role when there are multiple number of functional groups.
