Question
Question: Write the IUPAC name of the given compound? 
Solution
The name of alkene includes all substituents arranged in alphabetical order with their position and the name of the parent chain with the position of the double bond.
Complete step by step answer:
The IUPAC rules are used for naming the alkene.
The IUPAC rules for the naming of alkene are as follows:
Determine the longest carbon chain and determine the substituents attached to the chain.
If two same long chains are present then select the more substituted chain.
Give the numbering to the carbon atoms of the chains so all the substituents get the lowest numbering.
Arrange the substituents in alphabetical order with their position in the chain.
If the same substituent is present more than one, then add ‘di’ for two, ‘tri’ for three, and so on.
Select the longest parent chain as follows:
The longest carbon chain is of five carbon atoms. The name of five carbon atoms having alkene is ‘pentene’.
Determine the substituents as follows:
The longest chain has one bromine group and one methyl group.
Give the numbering to the chain as follows:
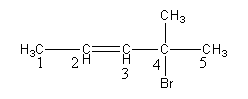
So, methyl and bromine groups are present at carbon-4 and double bonds are present at carbon-2.
‘ene’ suffix is used to show the presence of a double bond. The double bond is at carbon-2 so, the name of the parent chain is pent-2 -ene.
So, the name of alkene is of 4-bromo-4-methylpent-2-ene.
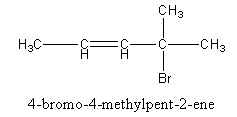
Therefore, the name of the structure is of 4-bromo-4-methylpent-2-ene.
Note: In alkene the longest carbon chain includes both the carbon atoms having double bonds. The simple five-carbon alkene is known as pentene where ‘pent’ indicates five carbons and ‘ene’ indicates a double bond. To specify the position of double bond the name is broken into two parts ‘pent’ and ‘ene’.
