Question
Question: Write the IUPAC name of the given compound – 
Solution
Try solving this question selecting the main chain, numbering it and choosing the root word. Then check it for being an alkane, alkene or alkyne. Finally write the name of the functional group (halogen in this case).
Complete answer:
Let us try to select a chain first. Going from left to right, we see that the chain consists of 3 carbons. Therefore, the root word will be ‘prop’, which means 3 carbon containing organic compounds.
Now, we can see that there is one double bond. Therefore, the compound is an alkene. So, its name becomes ‘prop’+’ene’, i.e. propene.
Keep in mind, while numbering any organic compound, we allot numbers in such a way that we get the lower number in prefix. Therefore –
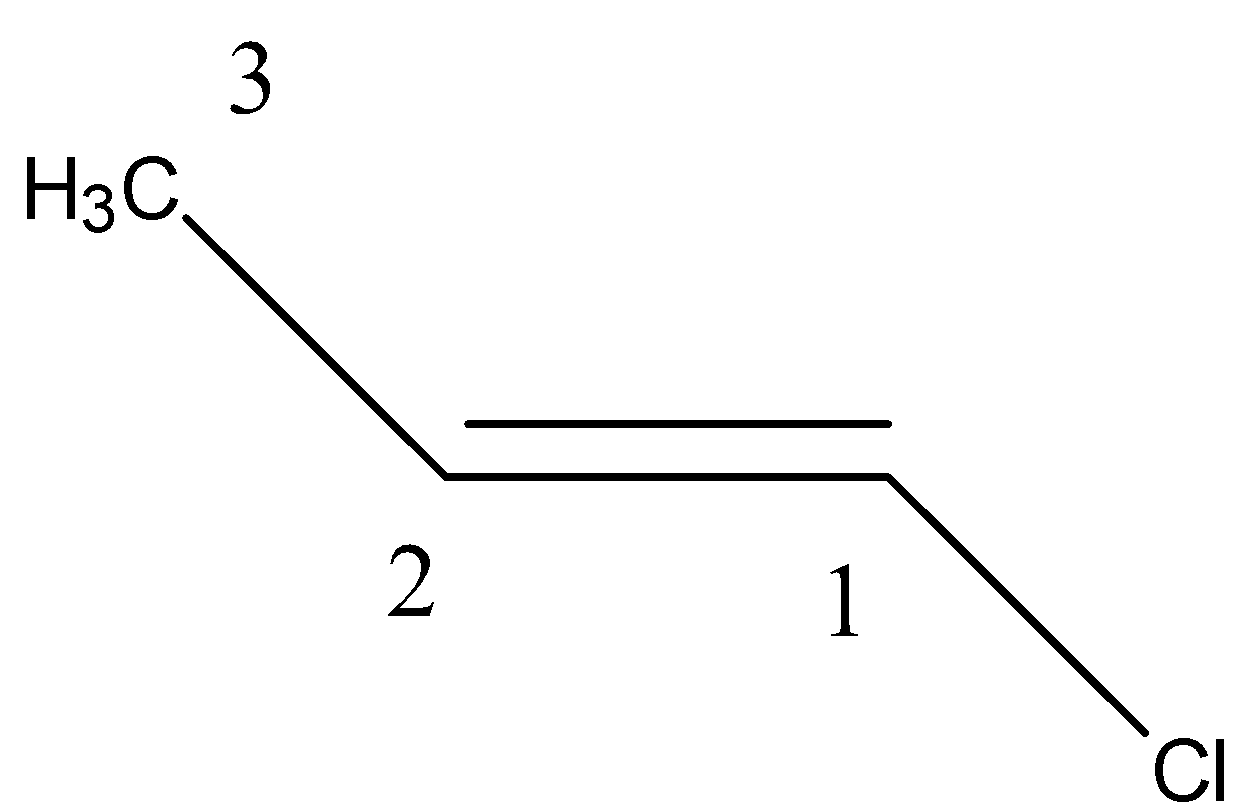
The compound, hence can be correctly written as prop-1-ene.
Now, let us see the functional group. As we can see there is a halogen in the compound, which is placed at the first carbon. Halogens are placed as a prefix. Therefore, Chlorine is written as a prefix ‘chloro-’.
Therefore, the name becomes 1-chloroprop-1-ene.
Lastly, let us check the configuration/geometrical isomerization of the compound. As we can see that the bulky groups are at other sides of the double bonds, we can conclude that it is a trans compound, which is denoted by ‘E’.
Therefore, the answer is – The IUPAC name of the given compound is (E)-1-chloroprop-1-ene.
Note:
The compounds which have the same molecular formula but are different in the relative spatial arrangement of atoms or groups in space are known as geometrical isomers. It is classified as cis and trans isomerism.
