Question
Question: Write the IUPAC name of the following compound. 
Solution
The answer to this question is based on the simple rules of IUPAC which includes the longest chain rule and also rule of suffixes and the multiple boned carbons are numbered first than the single bonded carbons.
Complete answer:
In our lower classes of chemistry, we have dealt with the basic concept of naming an organic chemistry that is based on the rule given by IUPAC (International union of pure and applied chemistry).
Now, we shall see the rules of IUPAC that will help us to number the given compound and then write its name accordingly.
- IUPAC rule for naming the aliphatic compounds states that the saturated hydrocarbons are considered as parent hydrocarbons and the other classes of the organic compounds are considered as their derivatives which is obtained by substituting one or more hydrogen atoms with functional groups.
- According to the longest chain rule, the continuous carbon chain containing maximum carbon atoms including even functional groups is selected and this is called as p[aren’t chain.
- According to the rule of suffixes, a primary suffix is added to the root word to indicate if the parent chain is saturated or unsaturated.
- Secondary suffix is added to the primary suffix to indicate the presence of a functional group.
- Since the above given compound does not have any functional group but is an unsaturated hydrocarbon, the parent chain must contain the carbon-carbon multiple bond, regardless of whether it is in the longest carbon chain or not.
Thus, the above given compound is numbered as,
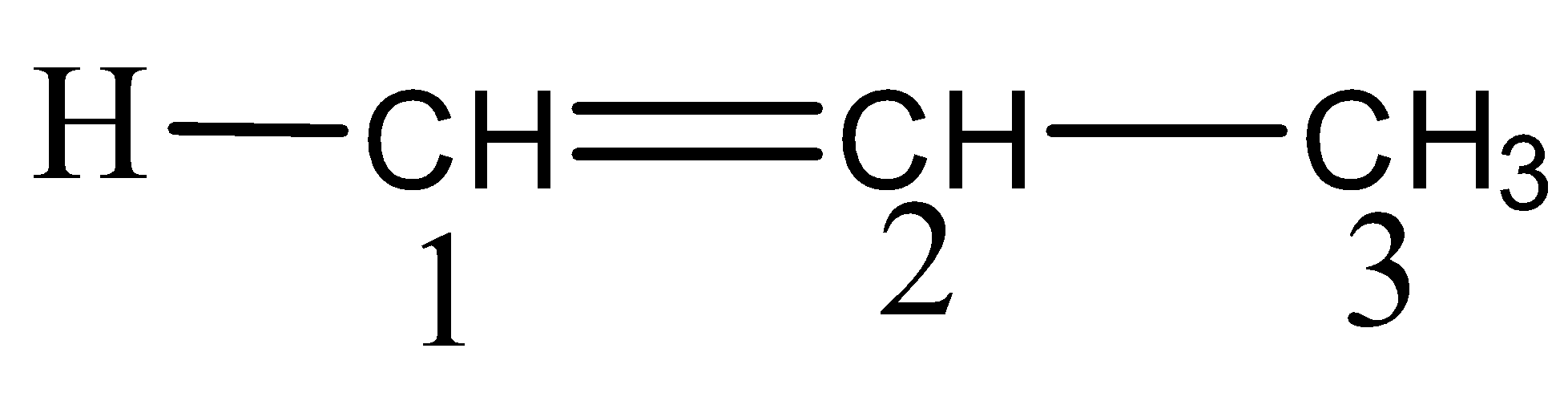
Here, there are three carbon atoms and hence the root word ‘prop’ is used along with the primary suffix ‘ene’ and since the double bond is numbered from 1, the name of this compound therefore, can be written as 1-propene.
Thus, the correct answer is, the IUPAC name of the given compound is 1-propene.
Note:
Note that propene is also written as propylene and do not be confused because both the name indicates the same compound and also the other name for this compound is methyl ethylene. This fact can help you to solve the question based on this type.
