Question
Question: Write the IUPAC name of the following compound: 
Solution
We know that in order to name organic compounds we must first know a few basic names of alkanes. In general, the base part of the name reflects the number of carbons in what we have assigned to be the parent chain. The suffix of the name reflects the type(s) of the functional group(s) present on (or within) the parent chain. Other groups which are attached to the parent chain are called substituents.
Complete answer:
Since, the given compound is
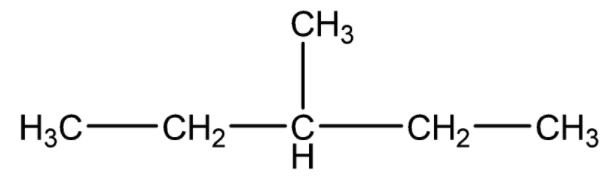
There are straight chain saturated hydrocarbons. There are five carbon atoms, so we will use ‘Pentane’. The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix –ane to -yl. Using the lowest locant rule, the branched methyl group is present at the carbon numbered 3.
So, the IUPAC name of the compound will be 3 - methylpentane.
Note:
The simplest hydrocarbons with all C-C bonds are alkanes. That is the reason they are called saturated hydrocarbons. The general formula for alkanes is CnH2n+2. In alkanes. all carbon atoms tend to complete their tetra valency by bonding with the same or different atoms and all the carbon atoms form single covalent bonds with other carbon atoms. The parent chain can be branched or unbranched and on the basis of that chemical and physical properties change. Alkanes are comparatively less reactive than hydrocarbons like alkenes, alkynes etc. because all carbon atoms are bonded with single covalent bonds in alkanes which are strong and less reactive in comparison to double or triple covalent bonds of alkenes and alkynes respectively.
