Question
Question: Write the IUPAC name of the following compound. 
Solution
IUPAC stands for International Union of Pure and applied chemistry, which is an international organization. In organic chemistry the chemical name of all the organic compounds is derived by following the nomenclature guideline of IUPAC.
According to IUPAC nomenclature, the name of an organic compound consists of three parts – prefix, word root and suffix.
The word root is the basic unit of the name, which tells us the number of carbon atoms present in the principle chain.
Suffix tell us the functional group present in the molecule, it may be the primary suffix (refers to the linkage between C-atoms) or secondary suffix (refers to the functional group present in the molecule), and secondary suffix is added after to the name of primary suffix.
Complete answer:
The naming of an organic compound in IUPAC nomenclature system follow mainly two rules, which are following –
(i) Selection of longest continuous parent chain. The selected longest continuous parent C- chain should contain a functional group or multiple bonds or substituents. Functional groups will give highest priority.
(ii) Numbering in the selected parent carbon chain. In selected parent C- chain numbering should be from that side from which functional group and multiple bond or substituent gets the lowest number. But the highest priority will be given to the functional group.
In this given compound there is no functional so numbering in the parent chain will be according to higher priority of substituent. Since, halogen(-Cl) and two methyl groups (-CH3) are present as a substituent so (-Halo) and (-alkyl) will be primary suffix. Since this compound is an open chain saturated hydrocarbon so -anewill be a secondary suffix, and this is a four carbon parent chain so -Bute will be the word root.
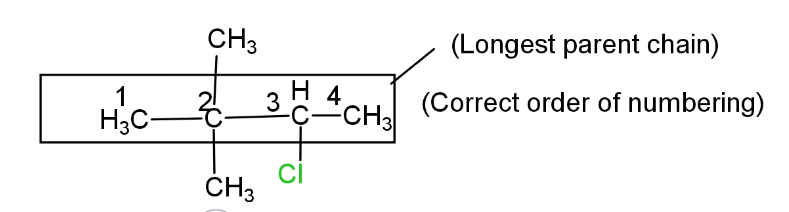
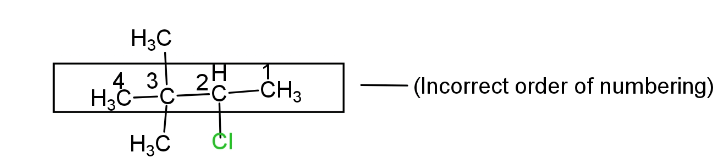
So the IUPAC name of the given compound will be 3-chloro-2,2-diemethylbutane.
Note:
If more than two substituents are present then minimum numbering should be done according to the lowest sum rule.
If more than two substituents are present in the parent chain then follow the alphabetical order during the naming of substituent.
