Question
Question: Write the IUPAC name for the compound \(C{{H}_{2}}OHC{{H}_{2}}OH\)....
Write the IUPAC name for the compound CH2OHCH2OH.
Solution
Visualize the structure of the given formula and try to name it by taking into consideration the parent alkane chain and then the substituents that are attached to it.
Compete step by step solution:
First, let us look at the structure of the given formula and then we will go about naming and numbering the atoms in the molecule. The structure is:
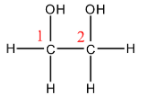
- Here, we can see the two atoms of carbon that are present in the parent alkane skeleton labelled as 1 and 2. This indicates that the entire name will have the root word as ‘ethan-’ and the entire name will be built around this.
- The order of numbering does not matter here because the molecule is symmetrical and will have the same substituent no matter which way we number it.
- We can see that there are alcohol groups present in the molecule, the suffix that we use for alcoholic functional groups is ‘-ol’. So, the name will be ethanol.
- We can see that there are two alcoholic functional groups so the name will end with ‘-diol’ indicating that there are two hydroxyl groups present. So, the name will be ethanediol.
- We need to add the location of the hydroxyl groups on the parent chain in between the name of the root chain and the suffix. Here, the groups are present on the first as well as the second carbon, we will have 1,2 in the middle.
From this, we can say that the overall name of the compound is ethan-1,2-diol.
Note: Remember that we should number the carbon atoms in such a way that the functional groups other than the hydrogen atoms attached to the carbon take the lowest possible number.
