Question
Question: Write the isomers of the compound having formula \[{C_4}{H_9}Br\] ....
Write the isomers of the compound having formula C4H9Br .
Solution
The isomers of any organic compound are the compounds having the same molecular formula yet different bond linkages. The number of atoms of each element remains the same but their positions can be changed to obtain different isomers.
Complete step by step answer:
The given compound contains four carbon atoms and a bromine substituent with the formula C4H9Br and is therefore named butyl bromide or bromobutane.
The molecular formulas of the organic compounds like alkyl halides give us the information about the type of alkyl chain and the nature of the halogen being used a substituent but fail to tell us the position of the substituent and whether or not the carbon chain is branched.
The simple straight chain butane has two different positions, as the first and fourth position and the second and third position are equivalent in nature. Thus, bromine can be attached to the first or the second position of the straight chain butane to give 1−bromobutane and 2−bromobutane respectively.
The butane chain can also get branched to form 2 - methylpropane which also has two different positions. The primary carbons and the tertiary carbon position. Thus, bromine can be attached to either of these positions to give 1 - bromo - 2 - methylpropane and 2 - bromo - 2 - methylpropane respectively.
⇒ Therefore, C4H9Br have four structural isomers that can be written as follows:

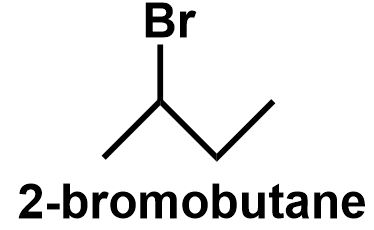
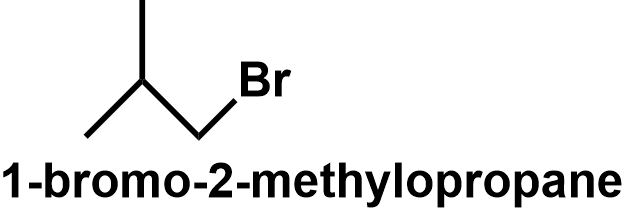
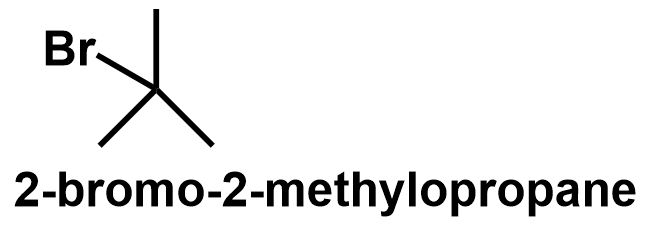
Note:
The formula does not change with the positions of bromine atoms; a bromine replaces a hydrogen in the parent chain and the number of hydrogens remain unchanged irrespective of which one is being replaced. The isomers are limited to four due to inability of the butane chain to branch further.
