Question
Question: Write the industrial production of ethanol with two uses....
Write the industrial production of ethanol with two uses.
Solution
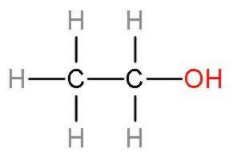
Ethanol consists of two carbon atoms and an alcohol group whose rest valencies are filled with hydrogen atoms. Ethanol is a colourless liquid. The structure of ethanol:
Molasses from which ethanol is produced from the juice extracted from sugarcane is boiled down until the sugars crystallise and precipitates out. The sweety syrup left out after crystallization is molasses.
- Ethanol is obtained commercially by fermentation.
Complete step by step answer:
Let us see the industrial production of ethanol which is from molasses:
- The sugar in molasses, sugarcane or fruits such as grapes is converted to glucose and fructose (C6H12O6) in the presence of an enzyme called invertase.
The reaction is C11H22O11+H2OInvertase2C6H12O6
- Glucose and Fructose undergo fermentation in the presence of another enzyme named zymase which is found in yeast. For making wine, grapes are the source of sugars and yeast. The reaction is C6H12O6zymase2C2H5OH+2CO2glucose ethanol .
- As grapes ripen, the quantity of sugar increases and yeast grows on their outer skin. When grapes are crushed, sugar and the enzymes come in contact and the process of fermentation starts. Fermentation takes place in anaerobic conditions meant in absence of air. Carbon dioxide is released during fermentation. If air gets into fermentation mixture, the oxygen gas oxidises ethanol to ethanoic acid which destroys the taste of alcoholic drinks.
Let us now discuss the uses of ethanol. The uses of ethanol:
(1)- The largest use of ethanol is as an engine fuel and fuel additive.
(2)- Ethanol increases the secretion of acids in the stomach.
Additional Information: Other uses of ethanol are:
(1)- It is used in medical wipes and in antibacterial hand sanitizers as an antiseptic for its antifungal effects.
(2)- It is used as a solvent in the paint industry.
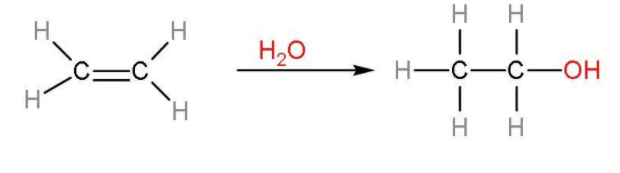
Note: The commercial alcohol is made unfit for drinking by mixing in it some pyridine (a foul smelling liquid) and copper sulphate (to give it a colour). It is called the denaturation of alcohols. The action of zymase is hindered once the percentage of alcohol formed exceeds 14%. Nowadays, ethanol is produced by hydration of ethenes. The process is