Question
Question: Write the formula of the following Diammine dichloro platinum (II)....
Write the formula of the following Diammine dichloro platinum (II).
Solution
Diammine dichloro platinum (II) is a neutral complex with the square planar geometry. It can exist in two forms namely cis-diammine dichloro platinum (II) called as cisplatin and trans-diammine dichloro platinum (II) complex called as transplatin.
Complete step by step answer:
The complex is a neutral complex because Pt will contribute 2+ to the complex, Cl will contribute 2- charge and NH3 will contribute 0 charge. Hence, the complex is a neutral complex.
In this Diammine dichloro platinum (II) complex, Platinum will be the central metal atom. It has four ligands surrounding it, namely two Cl atoms and two NH3 groups. Therefore, it will be having a square planar geometry. This complex can exist in two forms i.e. cis-isomer and trans-isomer.
cis-isomer is having the name cis-diammine dichloro platinum (II), which is also known as cisplatin and trans-isomer is having the name trans-diammine dichloro platinum (II), which is also known as transplatin.
Therefore, Formula for the Diammine dichloro platinum (II) complex is [Pt(NH3)2Cl2].
Additional information:
Structure of Diammine dichloro platinum (II) is given below:
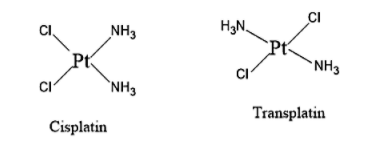
- When two Chlorine atoms are adjacent to one another in the Diammine dichloro platinum complex, it is called Cisplatin.
- When the two Chlorine atoms are opposite to one another in the Diammine dichloro platinum complex, it is called Transplatin.
Note: - Cis isomer of Diammine dichloro platinum (II) complex is having major importance compared to trans isomer.
- Cisplatin can be used as a medication to treat various cancers such as Bladder cancer, testicular cancer, breast cancer, ovarian cancer, etc.
- Use of cisplatin can also cause various side effects such as kidney problems, hearing impairments, bone-marrow suppression, heart problems.
- It can also be used in Auger therapy.
