Question
Question: Write the formula of monomers used in the preparation of dextron....
Write the formula of monomers used in the preparation of dextron.
Solution
Dextron is a polymer that is made up of two monomers and when these two monomers combine there is the elimination of water molecules, so it is a product of condensation polymerization. The monomers used for the preparation of dextron are acids, one acid contains 2 carbon atoms and the other contains 3 carbon atoms.
Complete answer:
We know that polymers are those compounds that have high very high molecular mass and a large number of molecules. These molecules are repeated which are known as monomers. As monomers are the smallest units.
The given polymer is Dextron. Dextron is a polymer that is made up of two monomers and when these two monomers combine there is the elimination of water molecules, so it is a product of condensation polymerization.
The monomers used for the preparation of dextron are acids, one acid contains 2 carbon atoms and the other contains 3 carbon atoms. So, one monomer is Lactic acid whose formula is C3H6O3 and the other monomer is Glycolic acid whose formula is C2H4O3.
The structural formula of Lactic acid is given below:
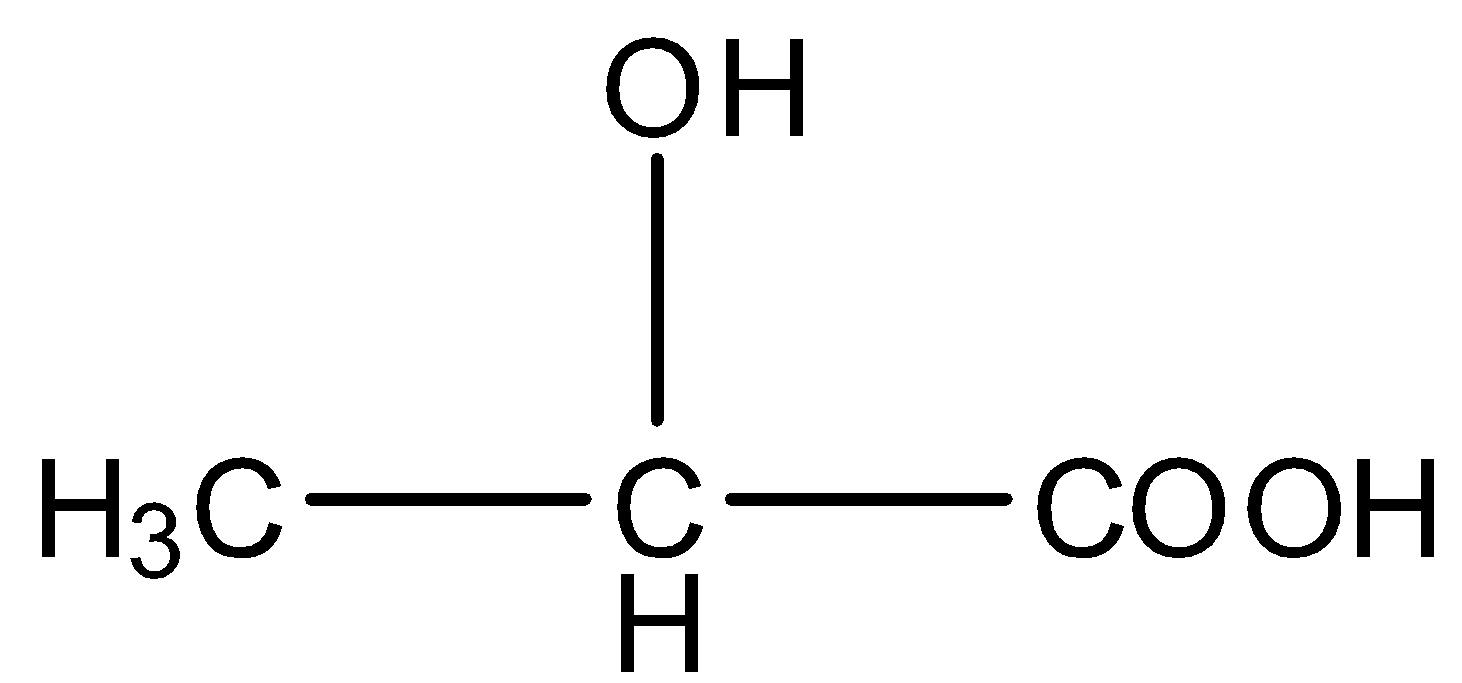
The structural formula of Glycolic acid is given below:
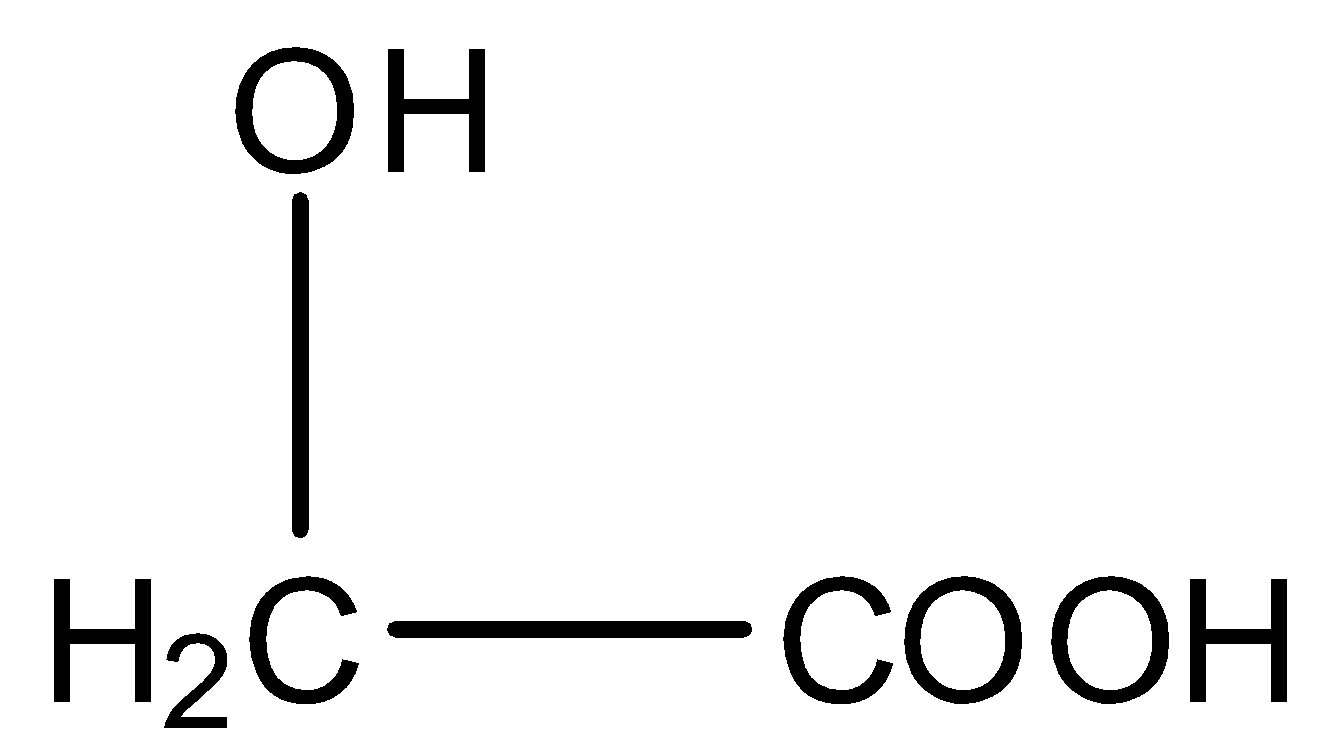
When they combine with each other, the OH group from the acid of one molecule and the H atom from the hydroxyl group of the other molecule will be removed, and this will form a dextron. The reaction is given below:
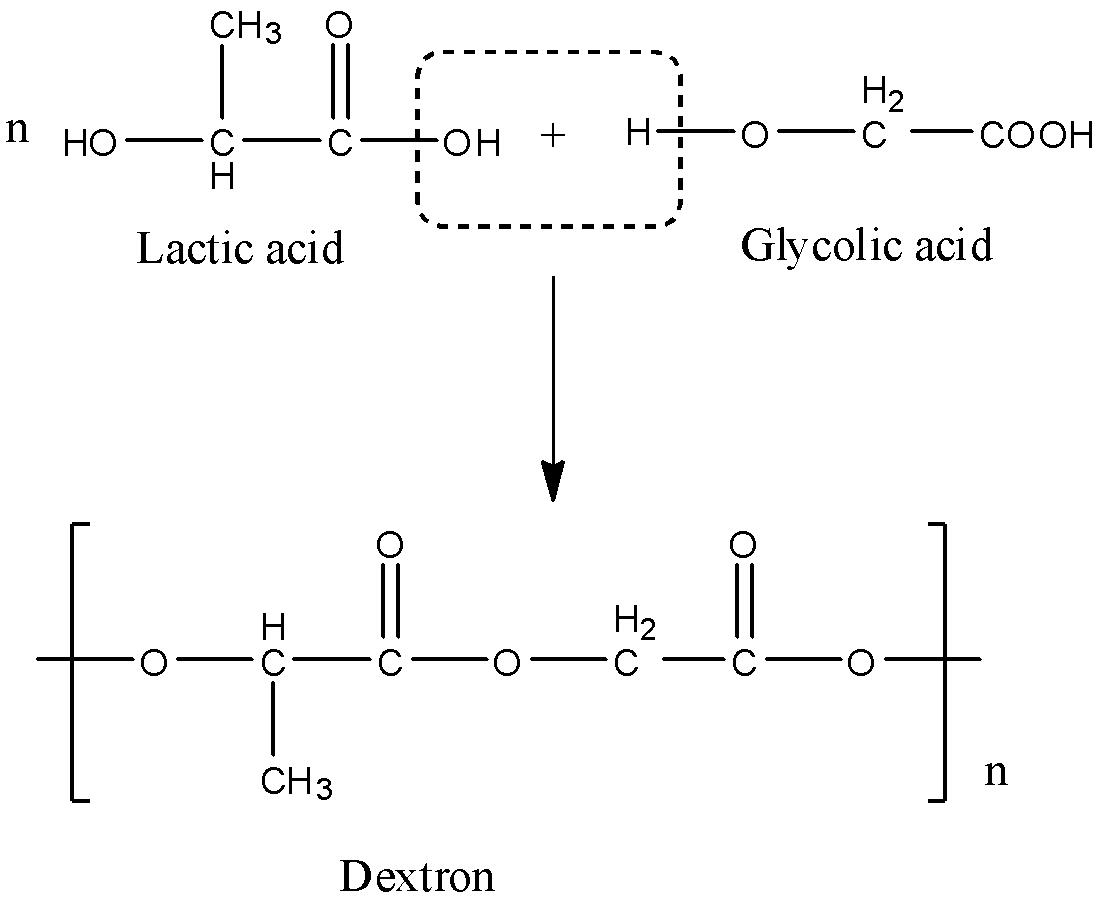
Note:
Polymers are formed by two processes, i.e., addition polymerization and condensation polymerization, if the polymer is formed without any loss of molecules then it comes under the addition polymerization.
