Question
Question: Write the equations involved in the following reactions: i.Reimer- Tiemann reaction ii.Williamso...
Write the equations involved in the following reactions:
i.Reimer- Tiemann reaction
ii.Williamson synthesis
Solution
To answer this question, you must recall both the reactions given in the question. The Reimer- Tiemann reaction is a reaction shown by phenols in presence of chloroform to give a hydroxy- benzaldehyde as the product. Williamson’s synthesis is the reaction between an alkyl halide (primary) and alkoxide ion which forms ether.
Complete answer:
(i) Reimer- Tiemann reaction:
Phenol is treated with chloroform and aqueous solution of a metal hydroxide. The chloroform group attacks the phenol ring as di- chlorocarbene which is a strong electrophile formed by the action of base on the chloroform molecule. An electrophilic substitution reaction takes place. Dichlorocarbene is a neutral but electron deficient species. A substituted benzyl chloride is formed after the attack which is turned into an aldehyde group by the hydroxide ions present in the solution. Generally, the aldehyde is added on to the ortho position of the hydroxyl group. A common compound formed using this reaction is salicylaldehyde.
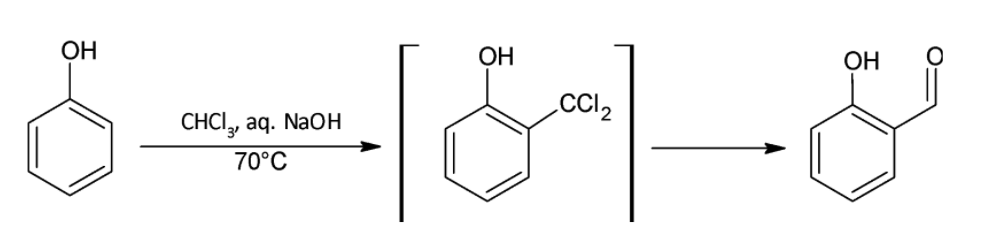
(ii) Williamson Synthesis
Williamson Synthesis also known as Williamson ether synthesis as the name suggests is a reaction for the preparation of ethers. In this process, an alcohol is converted to alkoxide anion by the sodium metal. Further, the alkoxide ion undergoes a nucleophilic substitution reaction by alkyl halides to form esters. It is used for the preparation of unsymmetrical ethers.
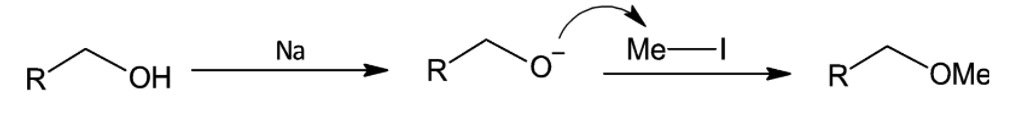
Note:
Williamson synthesis proceeds to bimolecular nucleophilic substitution reaction as the alkyl groups involved in the reaction are primary alkyl groups. This is because the carbocations that would be formed by unimolecular reaction, is not stable.
