Question
Question: Write the equation of the reaction of hydrogen iodide with: Methoxy benzene...
Write the equation of the reaction of hydrogen iodide with:
Methoxy benzene
Solution
Methoxy benzene is a type of alkyl aryl ether as it contains a bulky benzene ring along with −OCH3 group. Methoxy benzene is also called anisole. The reaction happens as a nucleophilic substitution reaction. The nucleophile generated is the iodide ion that attacks the least substituted alkyl group.
Complete answer:
Methoxy benzene called anisole is a type of aromatic ether having an alkyl group. It undergoes a nucleophilic substitution reaction with hydroiodic acid or hydrogen iodide. The nucleophilic substitution is of SN2 type where the nucleophile attacks the least substituted carbon. It happens in two steps where in the second step the SN2 reaction occurs.
The first step involves the protonation of the ether from the hydrogen iodide, a protonated ether is formed called as oxonium ion as:
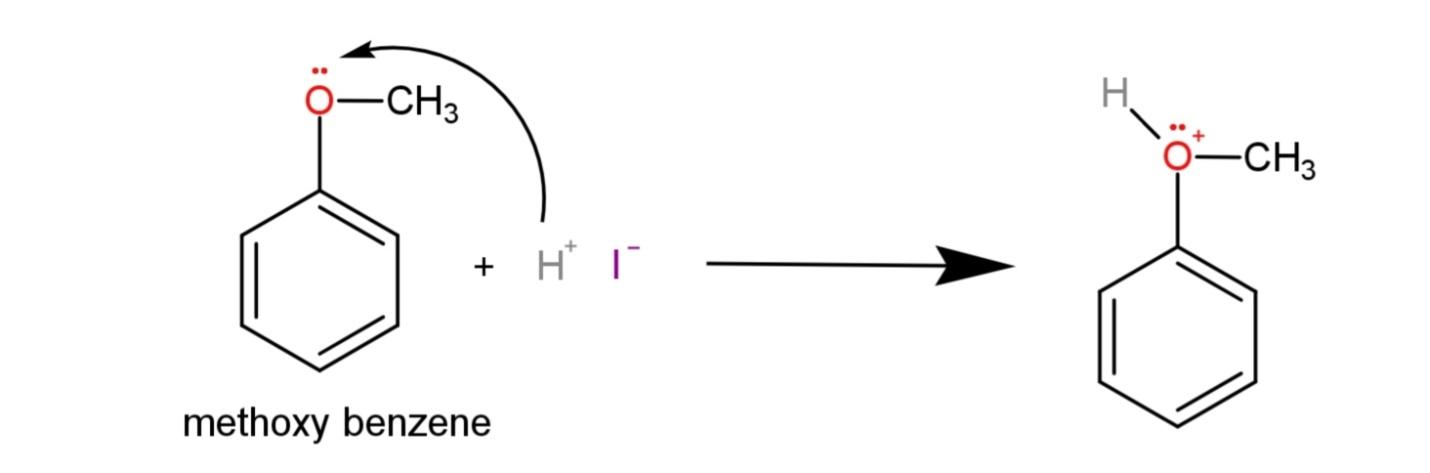
The second step involves the SN2 reaction where the iodide acting as a nucleophile attacks the less substituted methyl group and forms methyl iodide and phenol as:
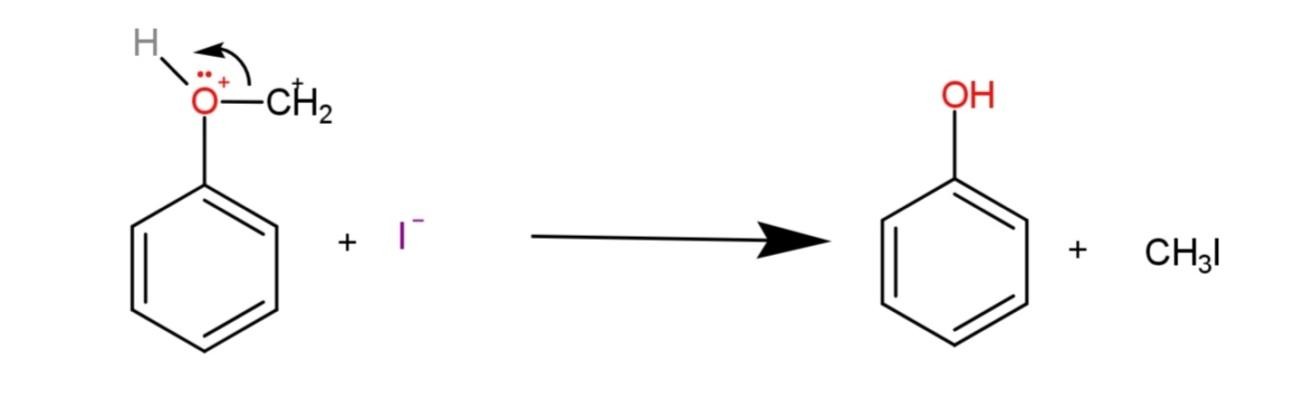
Hence, the reaction equation of hydrogen iodide with methoxy benzene is:
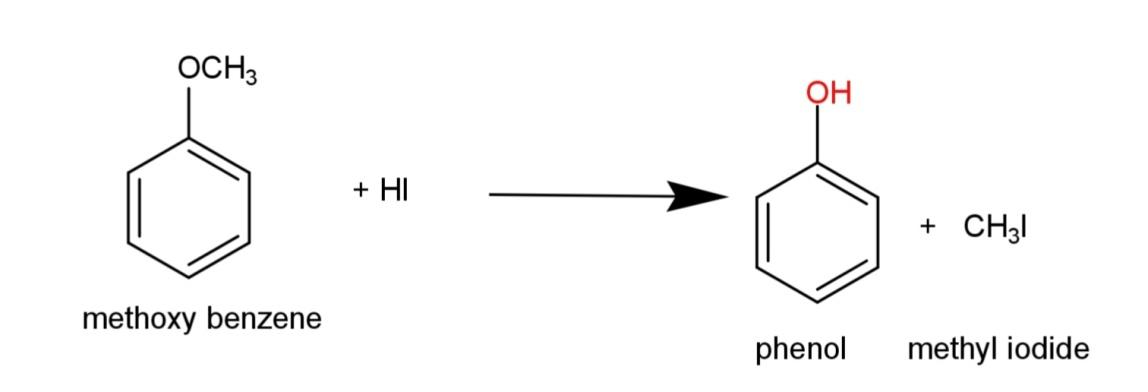
Note:
The methoxybenzene consist of two types of groups linked with the oxygen atom, one alkyl and the other aryl group. The nucleophile attacks the alkyl and forms methyl iodide as the aryl and the oxygen bond is more stable due to the delocalization of electrons of the ring and resonance, therefore a phenol molecule is formed from the aryl part.
