Question
Question: Write the electronic structure of propene, but-1-ene and but-2-ene....
Write the electronic structure of propene, but-1-ene and but-2-ene.
Solution
An electronic structure of given alkenes can be drawn from the molecular formula of the compound. The molecular formula of propene, but-1-ene and but-2-ene areC3H6,C4H8and C4H8respectively.
Complete step by step answer:
The molecular formula of propene is C3H6.The electronic structure of Propene is given below:
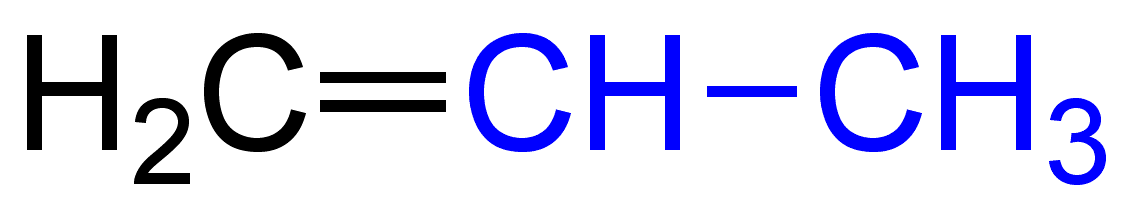
The molecular formula of But-1-ene is C4H8.The electronic structure of But-1-ene is given below:
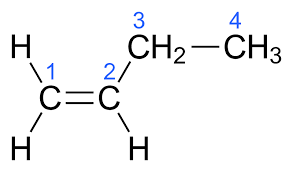
The molecular formula of But-2-ene is C4H8.The electronic structure of But-2-ene is given below:
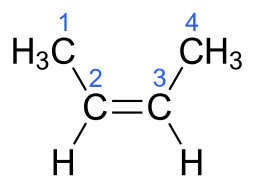
Additional Information:
-Following are steps to name an alkene following which we can draw the electronic structure of given alkenes.
-The ene suffix (ending) indicates an alkene or cycloalkene.
-The longest chain chosen for the root name must include both carbon atoms of the double bond.
-The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the centre of the chain, the nearest substituent rule is used to determine the end where numbering starts.
-The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator.
-If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator number.
Note:
But-1-ene and But-2-ene are structural isomers which differ in their structure but has same molecular formula. Since they differ in the position of double bond they are also known as position isomers.
