Question
Question: Write the electronic configuration of the Boron....
Write the electronic configuration of the Boron.
Solution
Boron belongs to the group no. 13 and it’s atomic no. is 5. So, it has 5 electrons. General valence electronic configuration of group 13 elements is ns2np1. To write the electronic configuration of boron, fill its 5 electrons in the orbitals arranged in order of increasing energies, according to Aufbau Principle.
Complete step by step solution:
An electron in an atom is characterised by 4 quantum numbers, and the principal quantum number (n) defines the main energy level known as the shell. Each shell consists of one or more subshells or sub-levels. Each subshell is assigned by azimuthal quantum no. (l) and lhave values from 0 to n−1 Also, each subshell has orbitals equal to 2l+1 . The distribution of electrons into orbitals of an atom is called its electronic configuration.
Boron has atomic no. 5 and it is represented by symbol the B. It belongs to group 13 of p-block. Group 13 elements outermost or valence electronic configuration is [E]ns2np1, where E is an inert gas configuration).
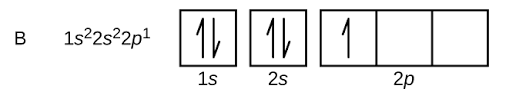
To find the electronic configuration of boron, fill 5 electrons of boron in orbitals according to Aufbau principle. Consequently, electronic configuration of boron will be :1s22s22p1.
Now, to write the noble gas electronic configuration of boron, we need to find the noble gas that comes before boron in the periodic table and it is helium ( electronic configuration of helium is 1s2).
Therefore, the noble gas electronic configuration of boron is [He]2s22p1. The 2 in 2s2and2p2 denotes the shell no (n) and shell number denotes the period no. to which the element belongs. Thus, boron belongs to period 2 of the periodic table.
Note: The orbital picture of boron can be represented as :
s- orbital can hold up maximum 2 electrons, p-orbitals can hold maximum 6 electrons.