Question
Question: Write the electronic configuration of \(M{n^{2 + }}\) and \(F{e^{2 + }}\)....
Write the electronic configuration of Mn2+ and Fe2+.
Solution
We are discussing the electronic configuration of some ions mentioned above and learning how to write it by its rules. Electronic Configurations means it is a standard notation used to describe the electronic description of an atom.
Complete step by step answer:
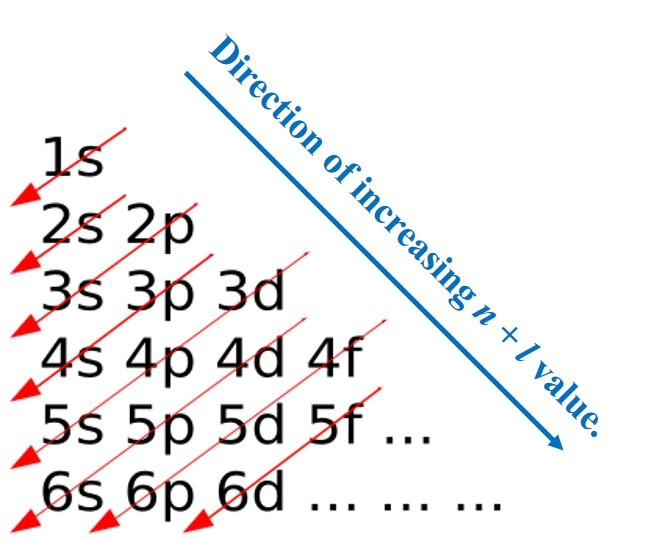
For using Electronic Configuration, Aufbau Principle (Aufbau is a German word for ‘Building-Up) is used which states that electrons occupy orbitals in order of increasing energy. The order of Energy is:
1s<2s<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p
Orbital: An orbital is a space where the probability of electrons is the highest.
There are 4 orbitals (s,p,d,f)
s is the orbital that has a maximum 2 electrons capacity.
p is the orbital that has a maximum 6 electrons capacity.
d is the orbital that has a maximum 10 electrons capacity.
f is the orbital that has a maximum 14 electrons capacity.
According to the Question,
Firstly, we can write the electronic configuration of Mn first its atomic number is 25.
1s22s22p63s23p64s23d5
After losing 2 electrons, it will become Mn2+.
Now, we can write the electronic configuration of Mn2+ ion
1s22s22p63s23p64s03d5
The electrons can be liberated from lower energy orbital (s Orbital) due to more energy gap between s orbital and p orbital and secondly, half-filled orbital and full filled orbital have higher energies and are mainly stable. So, from stable orbitals electrons cannot be released.
Now, We can write the electronic configuration of Fe first its atomic number is 26.
1s22s22p63s23p64s23d6
After losing 2 electrons, it will become Fe2+
1s22s22p63s23p64s03d6
The electrons can be liberated from lower energy orbital (s Orbital) due to more energy gap between s orbital and p orbital.
Note: Almost all the elements follow the same trend for writing electronic configuration. Sometimes when two sub-shells differ in the energies, an electron from the lower energy moves to higher energy. The orbitals in which the subshell is exactly half-filled or completely filled are more stable because of the symmetrical distribution of electrons.
