Question
Question: Write the electronic configuration based on molecular orbital theory for the molecular orbital of he...
Write the electronic configuration based on molecular orbital theory for the molecular orbital of helium He2 molecule. Calculate its bond order and comment on its magnetic property.
Solution
Molecular Orbital Theory is the quantum mechanical way of describing the electronic arrangement of the electrons in a molecule. The atomic orbitals combine to form low energy bonding and high energy antibonding orbitals.
Complete answer:
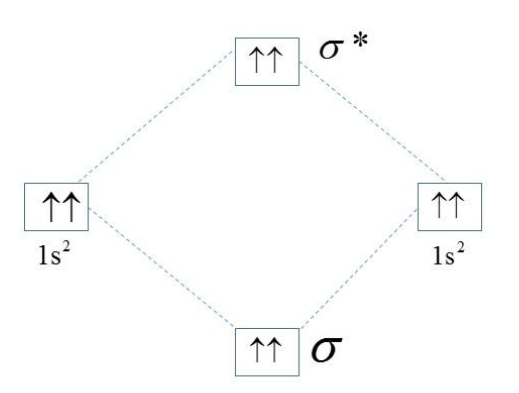
In the helium atom, there are two electrons in the valance 1s orbital. The other helium atom also has two electrons hence the linear combination of atomic orbitals (LCAO) of the 1s orbitals lead to the formation of a sigma bonding and antibonding orbitals. So one electron pair goes to the bonding while the other one goes to the antibonding molecular orbital. The bond order between any two atoms in a molecule has the formula,
B.O.=2No. of bonding electrons -No. of antibonding electrons .
For the helium atom, the number of bonding electrons and the number of antibonding electrons are the same and equal to two. Hence the bond order.
B.O.=22 - 2=20= 0.
Hence, there cannot be any bond between the helium atoms as the bond order between the atoms is zero. As there are no unpaired electrons in the atom of the helium, its magnetic moment is equal to zero.
Note:
According to the molecular orbital theory, the electrons in a molecule are arranged in three different molecular orbitals: the bonding, the anti-bonding, and the non-bonding molecular orbitals. The bonding and the antibonding orbitals can be further divided into sigma and pi molecular orbitals. These orbitals are as a result of the linear combination of atomic orbitals (LCAO) resulting from the bonds between different atoms.
