Question
Question: Write the electron dot structure of the ethyne molecule \(\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}...
Write the electron dot structure of the ethyne molecule C2H2 .
Solution
Lewis dot structure or the electron dot structure is a way of representation of valence electrons in the atom. These valence electrons are represented by the dot around the symbol of the element. The total number of valence electrons is equal to the number of dots in the structure. To determine the electron dot structure, determine the central atom (atoms which have high valence number and high electronegativity) and surrounded by the atom which has a low electronegativity. The ethyne has a molecular formula as C2H2 or CH≡CH .
Complete answer:
Let's draw a Lewis dot structure for ethane molecules. Ethyne is a saturated hydrocarbon. From its name it is clear that ethyne has two carbon atoms. Each carbon is sp hybridized and the molecular structure is C2H2 .
Follows the below step to determine the Lewis dot structure of the ethyne
Step 1) the first step determines the total number of valence electrons in the carbon atom. the electronic configuration of carbon is as follows,
C = 1s2 2s2 2p2
Here, carbon has a 4 valence electron.
Similarly, the electronic configuration of hydrogen is, H = 1s1
Here, carbon has a 1valence electron and requires one electron to attain the nearest noble gas configuration.
Step 2) In this step, draw a skeleton structure of the ethyne molecule. Connect all atoms through the single bond. The central atom will be the atom which has the highest number of valence electrons. Therefore, ethyne carbon is a central carbon atom. The skeleton would involve two carbon atoms surrounded by the two carbon atoms. The skeleton structure of ethyne is,
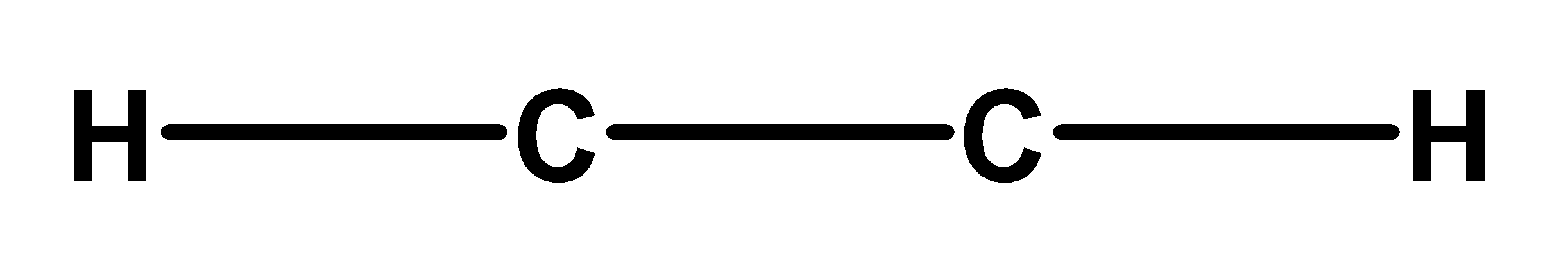
Step 3) In this step we will add all pairs of electrons on the atom. Each carbon atom has 4 valence electrons. Thus total valence electrons from two carbon atoms are Total valence e− = 2×(V.E. of C ) + 2×(V.E. of H ) ⇒ 2× 4 + 2×1 = 10
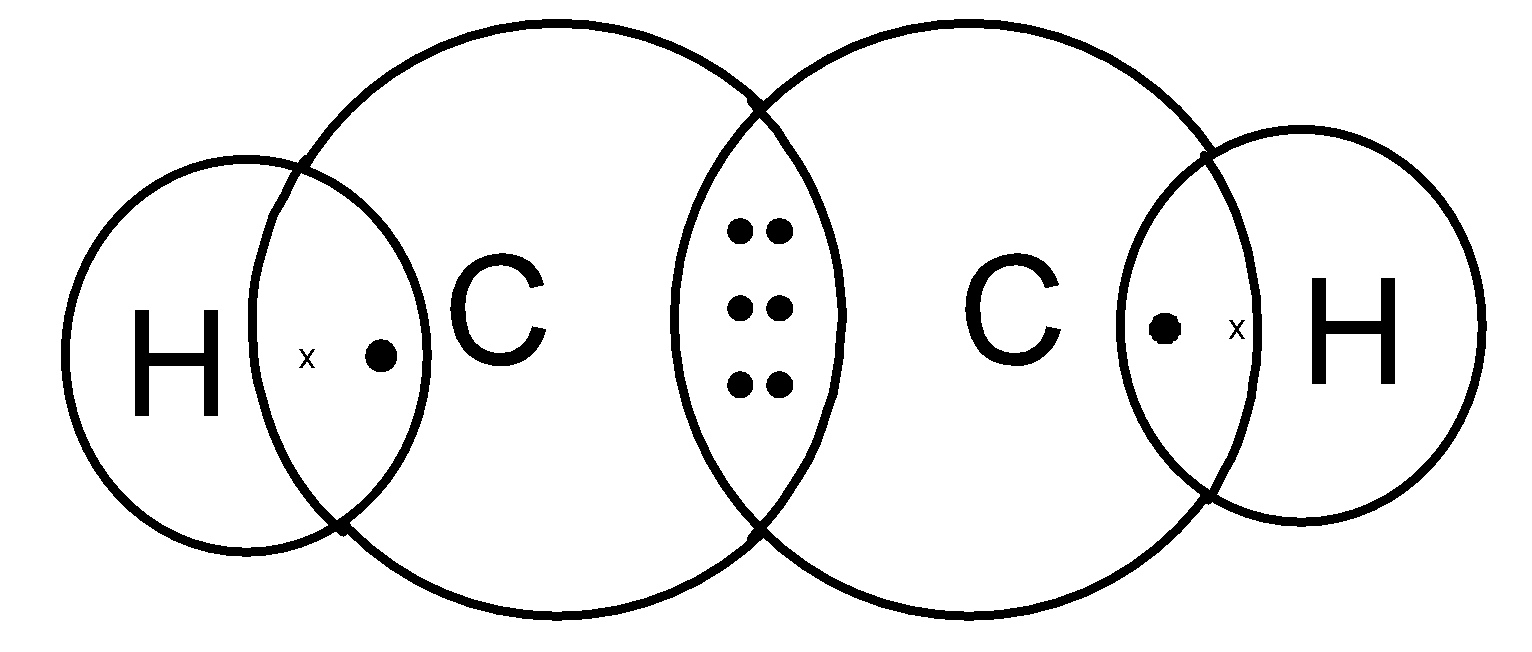
Thus we need to place 10 valence electrons on the ethyne molecule. Here six electrons are involved in the bond pairs. We need to place 4 electrons on the skeleton. The hydrogen atom has completed its octet. But each carbon requires 2 more electrons to complete its octet. thus add 4 electrons on the two carbon atoms to complete its octet. It is as shown below,

Ethyne structure can also be represented as,
**Note:**The Lewis dot structure does not explain the geometry of molecules or how the Bonds are formed, or how the electrons are shared between the atoms. The Lewis dot structure is a simple and limited theory based on the electronic structure and valence numbers.
