Question
Question: Write the different reactions taking place in the blast furnace during the extraction of iron from i...
Write the different reactions taking place in the blast furnace during the extraction of iron from its ore.
Solution
A blast furnace is a huge steel container lined with refractory bricks on the inner side. A blast of hot air at very high temperature is blown from the bottom and the materials to be heated are added from the top.
Complete step by step answer: 1) The temperature in the blast furnace varies from 1500K at the bottom to 500K in the upper part. We get iron oxide in the form of iron ore from mining. It has impurities and needs to be converted to iron metal for useful purposes. This is done in a blast furnace and the reactions taking place can be written as shown below.
2) The reactions taking place in the upper part of the blast furnace 500−800K :
1]3Fe2O3 + CO→2Fe3O4
2]Fe3O4+4CO→3Fe+4CO2
3]Fe3O4+CO→3Fe+4CO2
3) The reactions taking place in the lower part of the blast furnace 900−1500K :
1]C + CO2→2CO
2]FeO+CO→Fe+CO2 (Iron metal in molten state)
3]CaCO3→CaO+CO2
4]CaO+SiO2→CaSiO3 (Molten slag)
4) We get pure iron metal in the molten from at the bottom and the impurities are removed as slag. Additional information: In the blast furnace iron ore, limestone and coke (carbon) is added from the upper side and a blast of hot air is added from the bottom. The exhaust gases are pushed outside from an opening at the top.
5) The figure is shown as below:
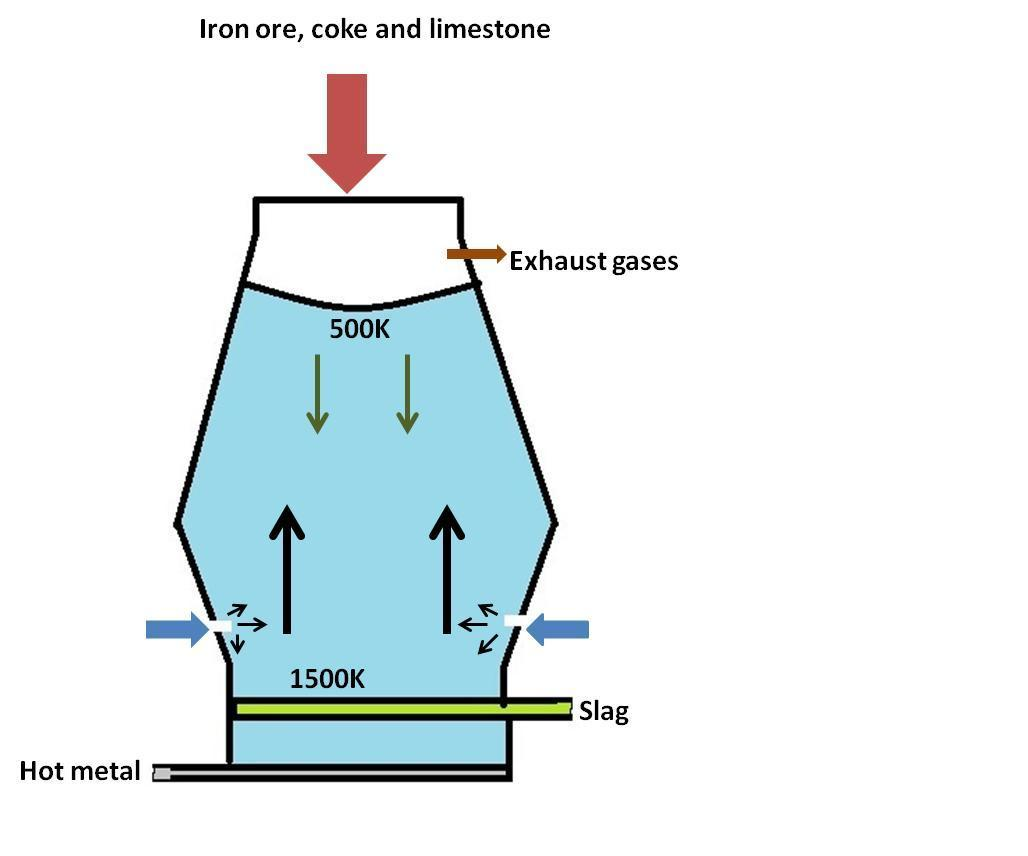
Note: Different reactions take place at different temperatures in the same blast furnace which are dependent upon the height at which reaction occurs. But at the end the product molten iron is obtained at the bottom. Oxygen is to be removed from the iron oxide to get pure iron metal and this process of removal of oxygen is called reduction. In this process iron oxide is reduced to iron and carbon is oxidized to carbon dioxide.
