Question
Question: Write the correct order of acidity: P. 
Q. 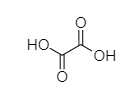
R. 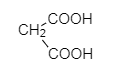
S. 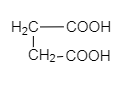
a.) P > Q > R > S
b.) Q > P > R > S
c.) Q > R > S > P
d.) S > R > Q > P
Solution
Hint: Acidity is the tendency of a compound to act as H+ donor. The carboxylic acid is acidic as it has hydrogen in the −COOH group. The compound whose carboxylate ion is the more stable will be more acidic.
Complete step by step solution:
Carboxylic acids are acidic in nature due to resonance stabilization of carboxylate ion and hydrogen of −COOH, which is found to be capable of ionization. As we know, oxalic acid easily releases its H atom to form CH3COO− ion whereas formic acid needs more energy. Hence, the Strength of oxalic acid is more than formic acid.
So, Oxalic acid (COOH-COOH) is more acidic than formic acid (HCOOH). Malonic acid, (COOH−CH2−COOH) is less acidic than oxalic acid because of the intervening presence of −CH2 group and succinic acid (COOH−CH2−CH2−COOH) which is much weaker than (COOH−CH2−COOH). Malonic acid due to the greater distance created by −CH2 group.
Hence, the order of acidity will be:
COOH-COOH > HCOOH > COOH-CH2-COOH > COOH-CH2-CH2-COOH
So, the correct order is QP R S and hence, the correct option is B.
Additional Information:
Carboxylic acids are important in the manufacture of greases, plastics, and crayons.
Carboxylic acids exhibit strong hydrogen bonding between the molecules.
Note: The possibility to make a mistake is that you can choose option D. COOH−COOH is more acidic than COOH−CH2−COOH due to +I effect which causes a decrease in acidity.
