Question
Question: Write the chemical equation of preparing acetone and write its four properties....
Write the chemical equation of preparing acetone and write its four properties.
Solution
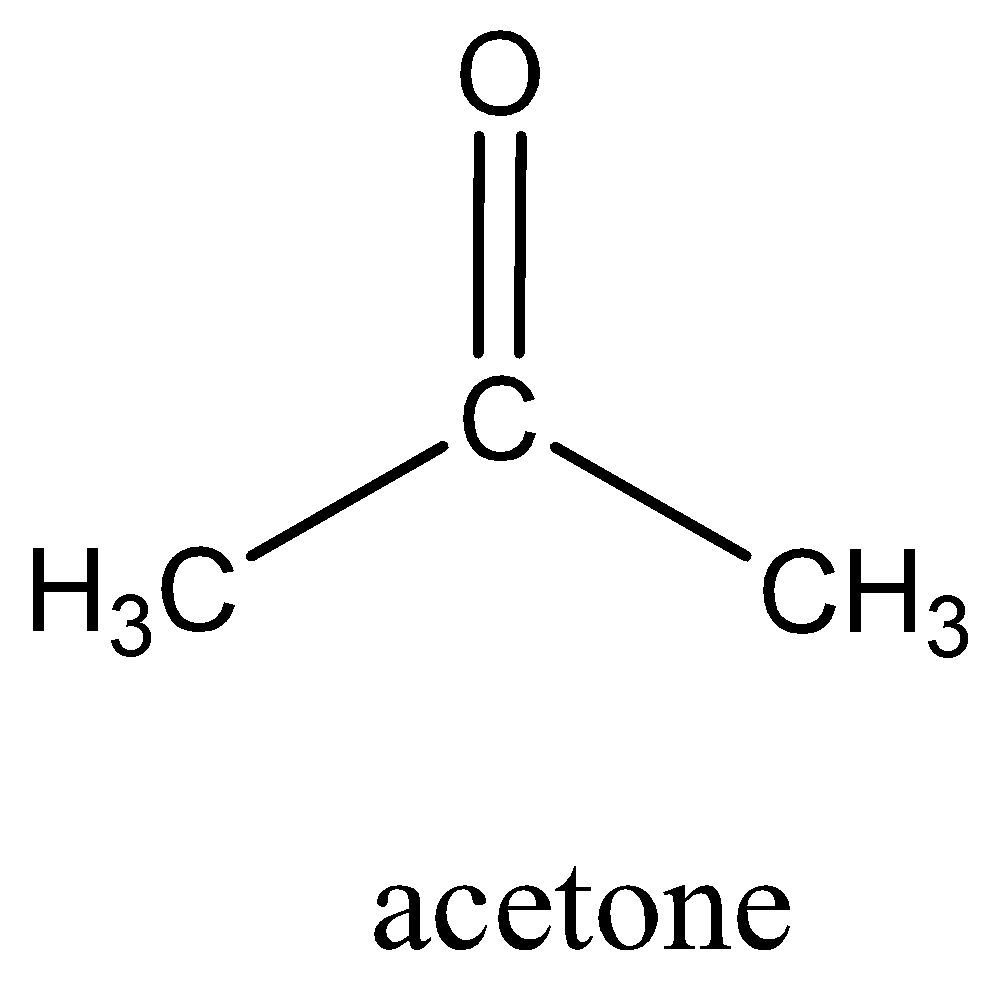
We must remember that the acetone (also known as propanone or dimethyl ketone), molecular formula is (CH3)2CO. It is an organic compound and smallest in ketone. It is mainly used as a solvent in organic chemistry and also home, industry, laboratory. It is colorless, and highly volatile and flammable liquid.
We can draw the structure of acetone as,
Complete step by step answer:
The chemical equation of preparing acetone is
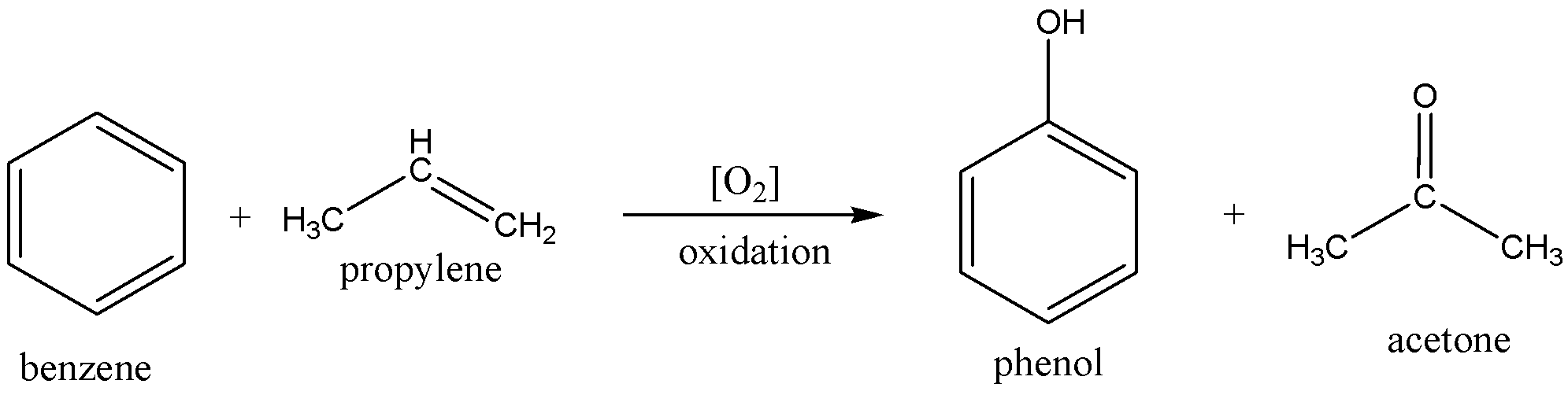 In the above preparation, the benzene molecule is alkylated with propylene and then the product cumene is oxidized to produce acetone and phenol.
In the above preparation, the benzene molecule is alkylated with propylene and then the product cumene is oxidized to produce acetone and phenol.
Now we can discuss about the properties of acetone as,
Keto/enol tautomerism
Structural isomers of a chemical compound which is readily interconverted and differ only in the position of protons and electrons. In tautomerism, the chemical compound of the carbon skeleton is unchanged. The keto (acetone) form of acetone is in equilibrium with the enol (prop - 1 - en - 2 - ol) isomer.
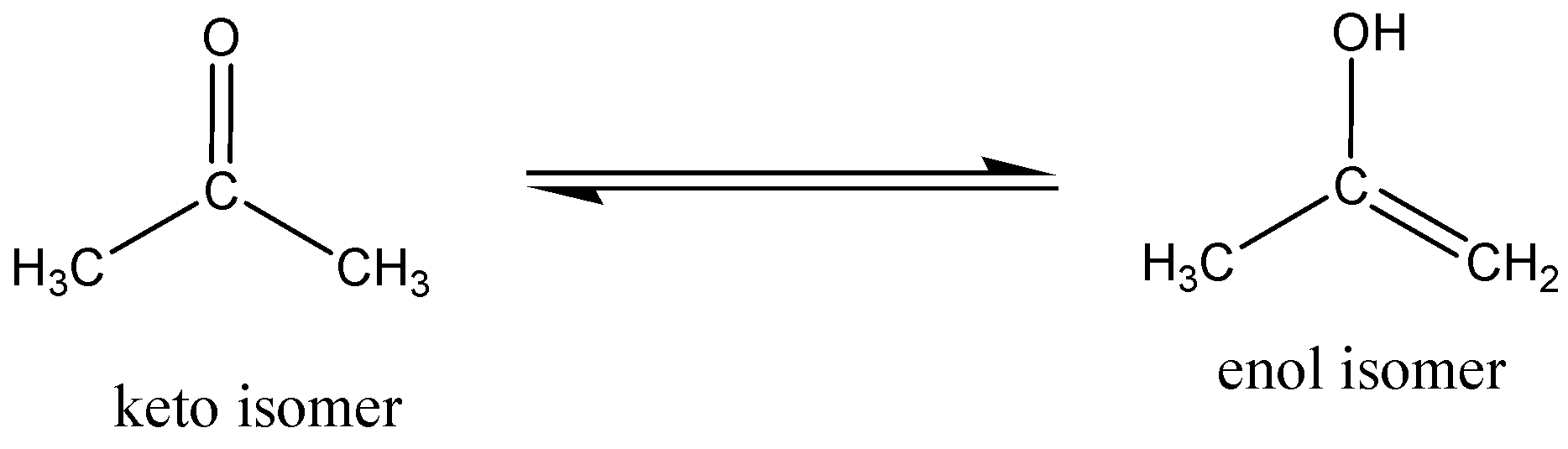
In ambient temperature, 2.4×10−7% of the molecules are in enol form in acetone.
Acetone is a polar solvent, and it is miscible with water due to the polar carbonyl group present in acetone.
Acetone is an active ingredient in paint thinner and nail polish remover.
Acetone easily dissolves another substance and it has low viscosity.
Note: In the human body acetone occurs naturally in the metabolism byproduct. Acetone is used in chemical peeling treatments and widely used in many general medicines, cosmetic products. For the production of methyl methacrylate and bisphenol A, it is used as a solvent.
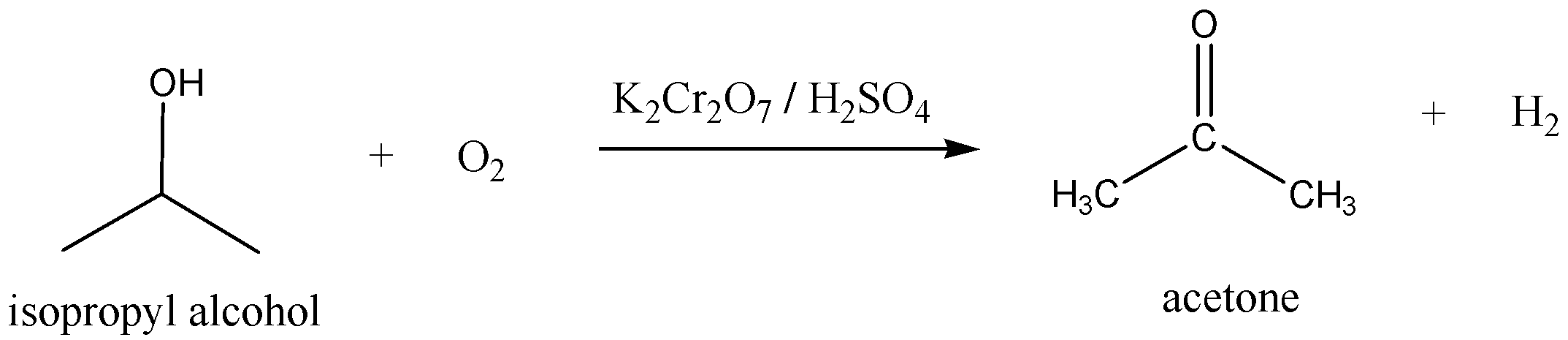
Another method to preparation of acetone is
We have to remember that the Isopropyl alcohol is oxidized by potassium dichromate (K2Cr2O7) with acid to give acetone and hydrogen. We can write the chemical equation for this reaction as,