Question
Question: Write the balanced equation for the chemical reaction in which Phosphorus burns in Chlorine gas to g...
Write the balanced equation for the chemical reaction in which Phosphorus burns in Chlorine gas to give out Phosphorus Pentachloride.
Solution
Hint: Remember that Phosphorus exists in its natural state as a P4 molecule. Also remember that the oxidation state of Phosphorus in Phosphorus Pentachloride is +5.
Step-by-Step Solution:
Let us study the chemical and physical properties of Phosphorus Pentachloride before studying its origination reaction i.e. the one concerning this question.
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, with the others being PCl3 and POCl3, and it is primarily used as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with HCl.
The structure of PCl5 is very much along the lines of the VSEPR theory and is observed to be as follows:
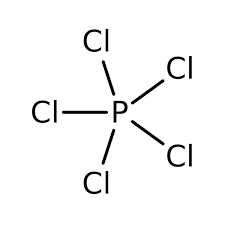
Now, while the primary method of the preparation of PCl5 involves the chlorination of PCl3; it can also be prepared by the burning of Phosphorus in the presence of Chlorine gas, the reaction for which can be written as follows:
P4+10Cl2Δ4PCl5
Which also happens to be the required chemical equation to complete this solution.
Note: In its most characteristic reaction, PCl5 reacts upon contact with water to release hydrogen chloride and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride (POCl3) whereas in hot water, it reacts to produce orthophosphoric acid (H3PO4).
