Question
Question: Write the atomic structure of the following carbon compound. 3,7 –dibromo-4, -6 dichloro – oct-5-e...
Write the atomic structure of the following carbon compound.
3,7 –dibromo-4, -6 dichloro – oct-5-ene-1, 2, diol
Solution
We know that in order to draw the structure of the carbon compound, we must first know a few basic names of alkanes. In general, first we will see the number of carbons in the name, in what we have assigned to be the parent chain. The suffix of the name reflects the type(s) of the functional group(s) present on (or within) the parent chain. Other groups which are attached to the parent chain are called substituents.
Complete answer:
Since, there is ‘oct’ in the name, which means there are eight carbons in the parent chain. And, as we know Numbers are assigned in the direction that gives the lowest numbers to the carbon atoms with attached substituents. Hyphens are used to separate numbers from the names of substituents;
The suffix ‘ol’ is for the alcohol and we see there are two alcohol attached to the parent chain at 1 and 2 carbon. It has ‘ene’ at carbon 5, which means it contains double bonds at carbon -5. The name ‘bromo’ and ‘chloro’ is there in the IUPAC name, so it means that there are two halogen atoms i.e., bromine and chlorine as we replace the -ine with -o (e.g., bromo, chloro, iodo). If there is more than one halogen atom then the prefix is used (e.g., 3,4-diiodo- or 1,2,2-trichloro-).
Also, the prefix ‘di’ means two. So, there are two bromines at 3 and 7 carbon atoms, two chlorine at 4 and 6 carbon atoms and two alcohol at 1 and 2 carbon atoms.
So, the structure of the carbon compound- 3,7 –dibromo-4, -6 dichloro – oct-5-ene-1, 2, diol is:
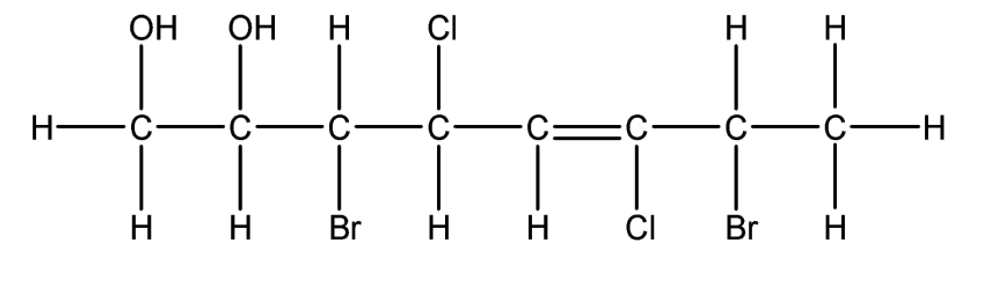
Note:
The IUPAC system requires first that we have names for simple unbranched chains, and second that we have names for simple alkyl groups that may be attached to the chains. We should note that the "ene" is the suffix for alkene. The symbol R is used to designate a generic (unspecified) alkyl group.
