Question
Question: Write the Arrhenius equation. Derive an expression for temperature variation....
Write the Arrhenius equation. Derive an expression for temperature variation.
Solution
Arrhenius deduced his expression in which he related the rate of the reaction to the effect of temperature which is known as The Arrhenius equation. This equation can be modified into a log form when two temperatures are given.
Complete step by step answer:
Arrhenius deduced his expression in which he related the rate of the reaction to the effect of temperature which is known as The Arrhenius equation.
The equation called the Arrhenius equation is usually written as k=Ae−Ea/RT where the pre-exponential factor A is a constant and is called frequency factor and Ea is called the activation energy, R is the gas constant and T is the temperature.
When we take logarithm on both sides, we get
lnk=lnA−RTEa
If the values of the rate constant at temperature T2 and T1 are k2 and k1 respectively, then we have,
lnk2=lnA−RT2Ea
lnk1=lnA−RT1Ea
So, subtracting the above equations, we get:
lnk2−lnk1=−RT2Ea−RT1Ea=RT1Ea−RT2Ea
Or we can write,
lnk1k2=REa[T2T1T2−T1]
And its log form is:
logk1k2=2.303REa[T2T1T2−T1]
To test the validity of the Arrhenius equation, let us consider the equation as:
lnk=−RTEa+lnA
Or it can be converted into:
logk=−2.303RTEa+logA
This equation can be written in the form of the equation of the straight line.
The equation of the straight line is: y=mx + c
So, in the straight line equation, when we plot a graph between y and x, we get m as the slop.
Similarly when we plot the graph for the equation logk=−2.303RTEa+logA:
logk is the y and T1 is the x, the validity of the equation is confirmed.
So, when we plot the graph we get m = 2.303R−Ea
So, the slope of the line = 2.303R−Ea
The graph of log k vs T1 is given below:
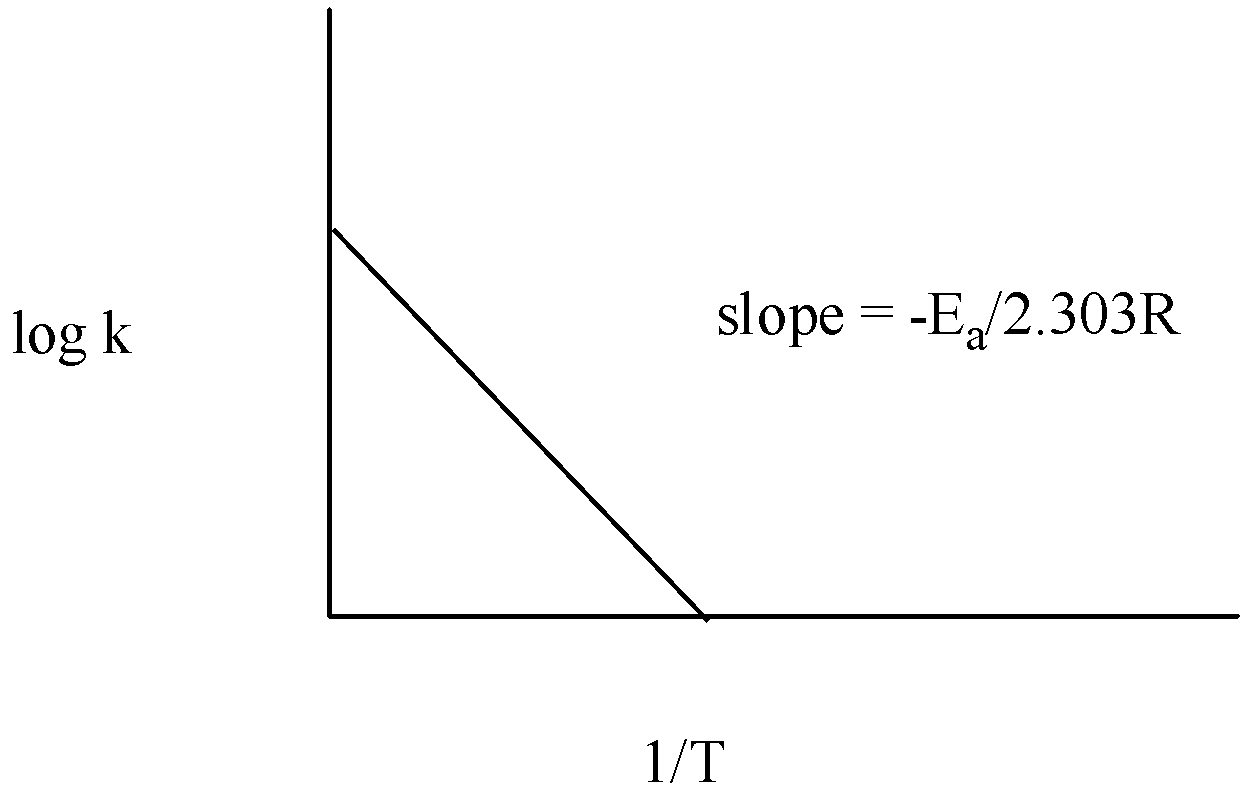
Thus, measuring the slope of the line, the value of Ea can be calculated.
Note: If we plot a straight line graph for the equationlnk=−RTEa+lnA, the y will be ln k and the x will be T1 so in this case, we get the slope of−REa. So, by converting any equation to the straight-line equation we can find the slope of the equation.
