Question
Question: Write one similarity between physisorption and chemisorption....
Write one similarity between physisorption and chemisorption.
Solution
Hint : The answer to this question lies in the question itself, think carefully about the definitions of both the terms and what exactly happens in both the processes and the answer will be clear.
Complete step by step solution:
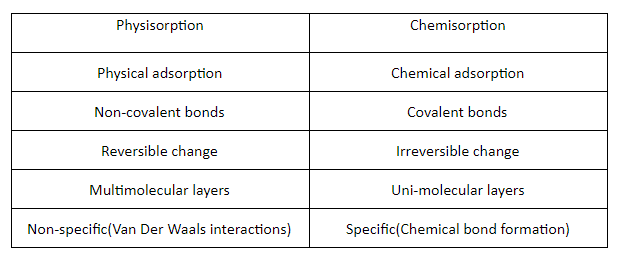
- Physisorption: It is basically physical adsorption of a certain substance onto the surface of another material in such a way that the chemical structure and composition of the surface that the substance has been adsorbed onto is not disturbed.
An example of physisorption can be seen in the reaction where alkenes are reduced using a metal catalyst. The catalyst is usually either platinum, palladium, or nickel. The hydrogen that is ultimately going to reduce the alkene gets adsorbed onto the surface of the metal, the surrounding alkene is reduced, and after the reaction is complete, the alkane detaches itself from the metal. Here, no chemical change occurs in the chemical composition of the metal.
-Chemisorption: It is a chemical kind of adsorption, a substance A adheres onto the surface of a material B with the help of covalent bonds that change the nature of the molecules on the surface of material B.
A very broad and macroscopic example of chemisorption is the rusting of iron. Oxygen adheres to the surface of iron, reacts with it to form iron oxide. Here, when oxygen gets adsorbed onto the surface of iron, it brings about a chemical change in the composition of iron. Hence, this is an apt example of chemisorption.
Both these definitions revolve around the same central theme of interaction between any 2 materials or substances on only the surface level. Chemisorption and physisorption both refer to adsorption and the interactions between molecules that are found on the surface of any material. In both the cases, the adsorbate (the substance that is adsorbed) and the adsorbent (the substance that adsorbs another) form some kind of bond with each other, may it be weak or strong, on the surface of the adsorbent.
The main similarity between physisorption and chemisorption is that they are both types of adsorption that only involve reactions on the surface of the adsorbent.Both increases with increase in the surface area.
Note : Please do not think that there is a small difference in both these terminologies, they are vastly different. The fact that they are surface interactions is one of the only similarities. Do not confuse any one of them with ‘absorption’, both of them are types of adsorption.
