Question
Question: Write Lewis symbols for the following atoms and ions: A.S and \[{S^{2 - }}\] B.Al and \[A{l^{3 ...
Write Lewis symbols for the following atoms and ions:
A.S and S2−
B.Al and Al3+
C.H and H−
Solution
Lewis structures are simplified diagrammatic representations of the valence shell electrons in a molecule. These symbols display how the electrons are arranged around the individual atom in a molecule. We represent electrons as dots and for bonding electrons, a line between two atoms is drawn.
Complete step by step answer:
A Lewis symbol can be drawn for a single atom, ions or a covalent compound as well. Typically, we represent only valence electrons in Lewis structures and avoid depicting non-valence electrons. Its main purpose is to fulfil the octet rule. It is denoted by an atomic symbol with the dots that represent the valence electrons around it.
Sulphur has an atomic symbol S and atomic number 16. Its electronic configuration is 1s22s22p63s23p4. So, it has 6 valence electrons. If we add two more electrons to it, S2− will be formed having 8 valence electrons. We can represent them as:

Aluminium has an atomic symbol of Al and atomic number 13. Its electronic configuration is 1s22s22p63s23p1. So, it has three valence electrons. If we remove these three electrons, Al3+ will be formed having no valence electrons. We can represent them as:

A Hydrogen atom has an atomic symbol H and its atomic number is 1. Its electronic configuration is 1s1. So, it has just one valence electron. If we add one more electron to it, H− will be formed having two valence electrons. We can represent them as:
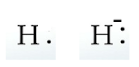
Note:
The Lewis dot structures do not interpret the geometry of the atoms or molecules and do not tell us how bonds are formed between two atoms or how the sharing of electrons results in covalent bonding.
