Question
Question: Write IUPAC names of the products obtained by the ozonolysis of 3,4-Dimethylhept-3-ene....
Write IUPAC names of the products obtained by the ozonolysis of 3,4-Dimethylhept-3-ene.
Solution
Ozonolysis is an organic reaction in which the unsaturated hydrocarbons that are alkenes and alkynes react with ozone which cleaves the double or triple bonds of the hydrocarbon. It may be oxidative or reductive depending on the reagent used which in return gives different products.
Complete answer:
Alkenes are unsaturated hydrocarbons which have at least one double bond between carbon atoms and alkynes are unsaturated hydrocarbons which have at least one triple bond between carbon atoms. These hydrocarbons are unsaturated because all carbon atoms do not have the maximum number of hydrogen atoms it can carry due to the presence of double or triple bonds. Triple bonds are stronger than double bonds because they are bonded due to interactions between six electrons and it has one sigma bond and two pi bonds.
Ozonolysis reaction is an organic reaction of unsaturated hydrocarbons with ozone. The double or triple bonds of the unsaturated hydrocarbons are cleaved by ozone on reaction. The bonds are replaced by carbonyl groups. It is also known as ozonolysis-reduction because an appropriate reducing agent is required to break down the double or triple bond.
Depending on the reagents if a reduction work up is done then either alcohol or carbonyl products are formed and if an oxidation work up is done then either carboxylic acid or ketone products are formed.
On ozonolysis of 3,4-Dimethylhept-3-ene with O3 , the double bond or triple bond is cleaved. After cleavage of bond Zinc dust is used to prevent further oxidation of the compound.
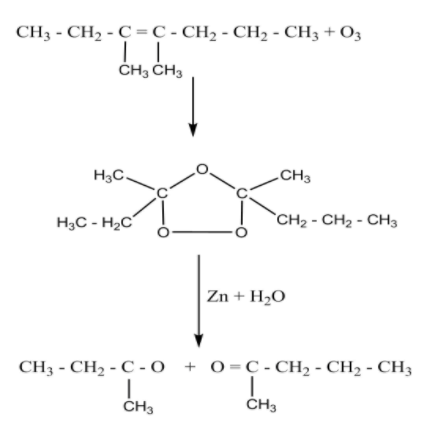
Therefore, the IUPAC names of the products after ozonolysis of 3,4-dimethylhept-3-ene are Butan-1-one and pentan-2-one.
Note:
Ozonolysis has many applications especially in organic chemical research. It is also used in synthesizing some particular natural products and is used in industries too. Zinc prevents the compound from making further bonds with oxygen and thus stops the reaction process.
