Question
Question: Write IUPAC name of the following: 
Solution
First identify the main functional group present by seeing which one has the highest priority and do the naming of the parent carbon chain. Then do the numbering for the substituent so that they lie nearest to the functional group carbon.
Complete step by step answer:
-The first step here is to identify the longest carbon chain and the most prior functional group present.
The 2 functional groups attached to benzene are: a ketone (C6H5−CO−CH3) and an ether (−OCH3). Of both ketone and ether we know that ketone is much more prior than ether and so it will be a major functional group while ether will act as substituent to the benzene ring.
Also for compound (C6H5−CO−CH3) the longest carbon chain is of 2 carbons (−COCH3) and benzene ring acts as substituent. So, its name is 1-phenylethanone.
But here in the given compound we have an ether substituent (−OCH3) of name methoxy which is attached to the substituent benzene ring. So, the name of the substituent would be: 2’-methoxyphenyl.
The numbering is done as follows:
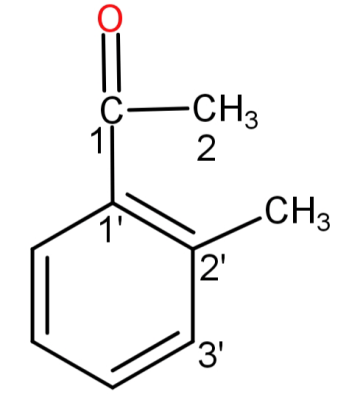
This substituent is attached to the carbon number 1 of the main chain so the IUPAC name would be: 1-(2’-methoxyphenyl)ethan-1-one. This compound is also known as 2-methoxyacetophenone and o-methoxyacetophenone (common names).
Hence the IUPAC name of the compound is: 1-(2’-methoxyphenyl)ethan-1-one.
Note: The priority order of the functional groups is: carboxylic acid > sulfonic acid > ester > acid halide > amide > nitrile > aldehyde > ketone > alcohol > thiol > amine > ether. If any compound has two or more functional groups the numbering is always done on the basis of the priority of the functional groups.
