Question
Question: Write IUPAC name of isobutane....
Write IUPAC name of isobutane.
Solution
We know that all alkanes do not have linear-chain. Some alkanes are said to be in branched forms. We have to know that branched alkanes differ from straight-chain alkanes in the chains of carbon atoms which displace a few hydrogen atoms found along the chain. Substituent is the term given to groups (or) atoms that replace hydrogen in an alkane.
Complete step by step answer:
We have to know that naming a straight chain alkane is much simpler than naming a branched alkane. We will discuss some of the steps to obtain the IUPAC names of substituted alkanes.
1.The first step is to count the longest chain of carbons. The longest chain should be continuous.
2.In the second step, we have to count the number of carbons found in the chain beginning with the side which is nearby to the branch. We call the longest chain present in branched alkane using the term “parent chain”.
3.In the third step, we should count the number of carbons present in each branch. The carbons found in branches are known as alkyl groups and they have one carbon less than alkane group. For example, if there is one carbon found, we call the group a methyl group, if it’s two carbons, then it is ethyl and so on.
4.We should attach the number of carbon from each substituent branch to the front of the alkyl group name. For example, if a group having two carbons is linked to a fourth carbon present in the chain, then we call the group 4-ethyl.
5.In the fifth step, we should check for repeated alkyl groups. If we see multiple groups found with the same number of carbons branched off the parent chain, we must not repeat the name. In such instances, we can use prefixes such as di-, tri-, and tetra-.
6.In the sixth step, we should place the names of the substituent groups before the name of the parent chain in alphabetical order. We need not alphabetize the prefixes like di-, tri-, and tetra-.
Now coming back to the question, we have to first draw the structure of isobutane and count the number of carbon present in the longest chain.
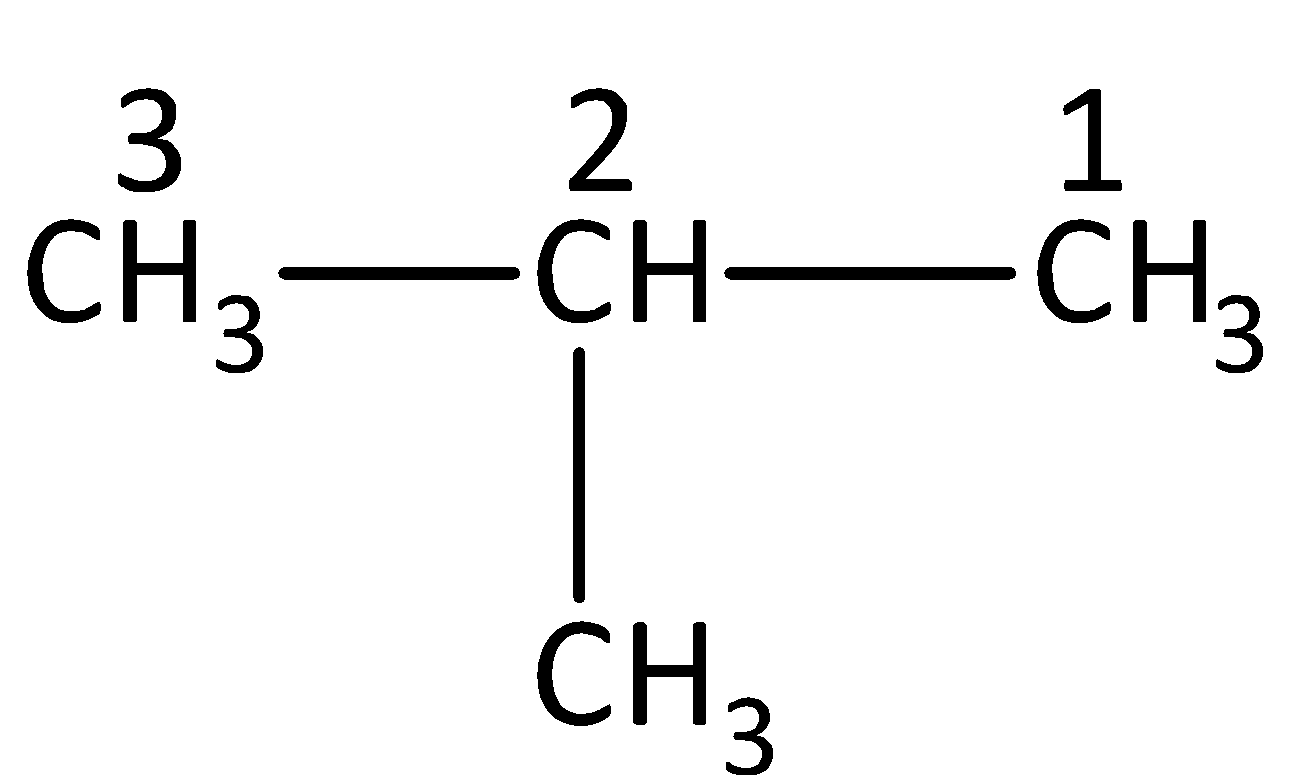
From the structure of isobutane, we have identified that the longest carbon chain consists of three carbon atoms and therefore, the parent chain is propane.
Now coming to the branching chain, we can see that an alkyl group is present as substituent and it contains one carbon atom, so the branched alkyl chain has a methyl group.
We can see that the methyl group is attached to the second position of the parent chain, so we have to name it as 2-methyl.
The IUPAC name of isobutane is 2−Methylpropane.
Note: We could obtain isobutane by isomerization of butane. It has a molecular formula of HC(CH3)3 and it is also known as methylpropane. The simplest alkane that contains tertiary carbon atoms is isobutane. We can use this molecule as a precursor in the petrochemical industry.
