Question
Question: Write down the structures of stereoisomers formed when cis-2-butene is treated with bromine....
Write down the structures of stereoisomers formed when cis-2-butene is treated with bromine.
Solution
In order to draw the stereoisomers at first we need to know the formula of the parent molecule. From the molecular structure of the parent molecule we can derive the stereoisomers. In the answer which is mentioned below we have been classifying the various ways in which the ions participating in the chemical reactions can be rearranged, and the final products are also mentioned.
_Complete step by step solution: _The molecular formula of cis-2-butene is C4H8. From the molecular formula of cis-2-butene, we can derive the molecular structure of cis-2-butene
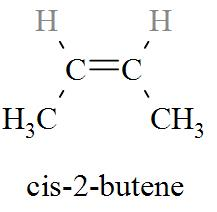
When cis-2-butene is treated with bromine, Bromonium ion is formed. The chemical reaction is shown in details in the following diagram.
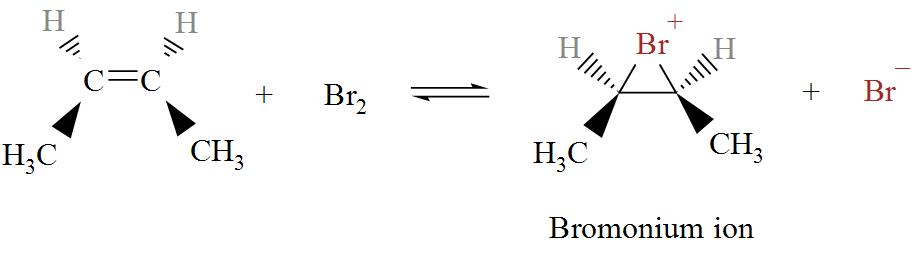
In the following diagram we can see bromine ion attacking the Bromonium ions in two possible ways.
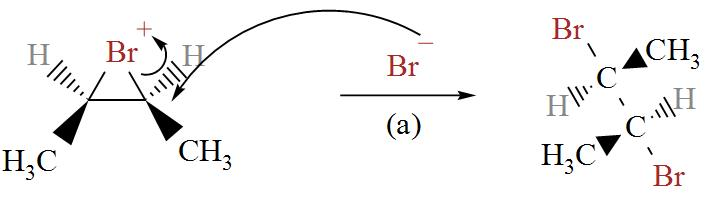
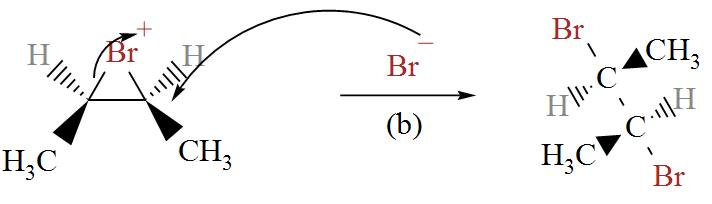
From here, we get two stereoisomers of cis-2-butene, (2R, 3R) and (2S, 3S).

These are the end-products when cis-2-butene reacts with Bromine.
Note: In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of the atoms in space.
Structural isomers are basically classified as those compounds which differ in their connectivity between the constituent atoms that make up the component. However the stereoisomers should not be confused with them because they have the exact same molecular formula. The only thing that differs is the arrangement of the atoms in the three-dimensional space.
