Question
Question: Write down the structural formula of ethane and ethene....
Write down the structural formula of ethane and ethene.
Solution
The organic compounds which are composed of only hydrogen and carbon atoms are known as hydrocarbons. In the compounds, the hydrogen atoms are bonded to carbon atoms via covalent bonds and carbon atoms in the compounds show catenation i.e., have a tendency to form long chains and ring like structures.
Complete answer: Hydrocarbons are classified into two types which are discussed below.
Saturated hydrocarbons:
These hydrocarbons are also known as alkanes. In these hydrocarbons, a carbon atom is bonded to another carbon atom via a single bond and each carbon atom present in the structure of alkane is sp3 hybridized. It has a tetrahedral geometry and the general formula for alkanes is CnH2n+2 where n denotes the number of carbon atoms present in alkane.
For example: Ethane- There are two carbon atoms in ethane i.e., the value of n for ethane molecules is 2. So, the formula for ethane =C2H2×2+2.
∴Structural formula of ethane =C2H6
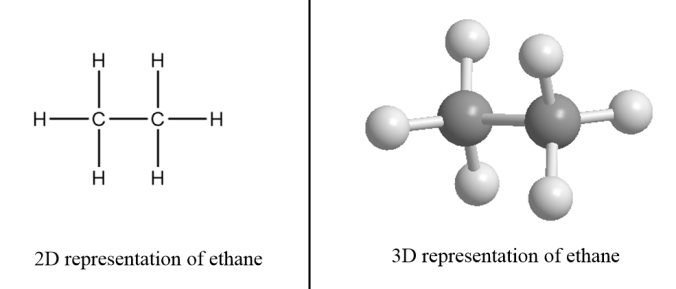
Unsaturated hydrocarbons:
These are further divided into two types i.e., alkenes and alkynes.
Alkenes- These are the hydrocarbons in which at least one carbon atom is bonded via double bond in the structure and that carbon atom is sp2 hybridized. It has a planar geometry and general formula for alkenes is CnH2n where the value of n=2,3,4⋅⋅⋅ i.e., the compound must consist of at least two carbon atoms to form an alkene.
For example: Ethene- There are two carbon atoms in ethene i.e., value of n for ethene molecule is 2. So, the formula for ethene =C2H2×2.
∴Structural formula of ethene =C2H4
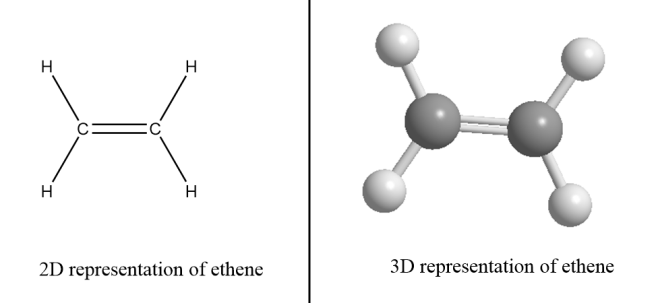
Alkynes- These are the hydrocarbons in which at least one carbon atom is bonded via triple bond in the structure and that carbon atom is sp hybridized. It has a linear geometry and general formula for alkynes is CnH2n−2 where the value of n=2,3,4⋅⋅⋅ i.e., the compound must consist of at least two carbon atoms to form an alkyne.
For example: Ethyne- There are two carbon atoms in ethyne i.e., the value of n for ethyne molecules is 2. So, the formula for ethyne =C2H2×2−2.
∴Structural formula of ethene =C2H2
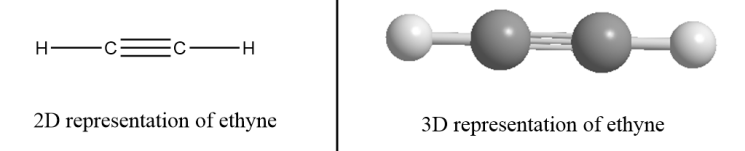
Hence, the structural formula of ethane and ethene is C2H6 and C2H4 respectively.
Note:
It is important to note that a series of compounds which consist of similar chemical properties and functional groups but differ by CH2 group in the structure is known as a homologous series. The general formula given to alkanes, alkenes and alkynes is based on the concept of homologous series.
