Question
Question: Write down the possible isomers and give their IUPAC names using the formula \({C_4}{H_{10}}\) ....
Write down the possible isomers and give their IUPAC names using the formula C4H10 .
Solution
The compounds having the same molecular formula but different structural formulas are called isomers of each other and the phenomenon is called Isomerism.
C4H10 has only two isomers - n-butane and isobutene.
Complete step by step answer:
Butane is an alkane with four carbon atoms. Its molecular formula is C4H10 . Its two isomers are n-butane and isobutane or 2 - methylpropane.
n-butane: n butane or normal butane or butane is a colourless gas with a faint petroleum like odour. Its average mass is 58.122 Da and monoisotopic mass is 58.07251 Da.
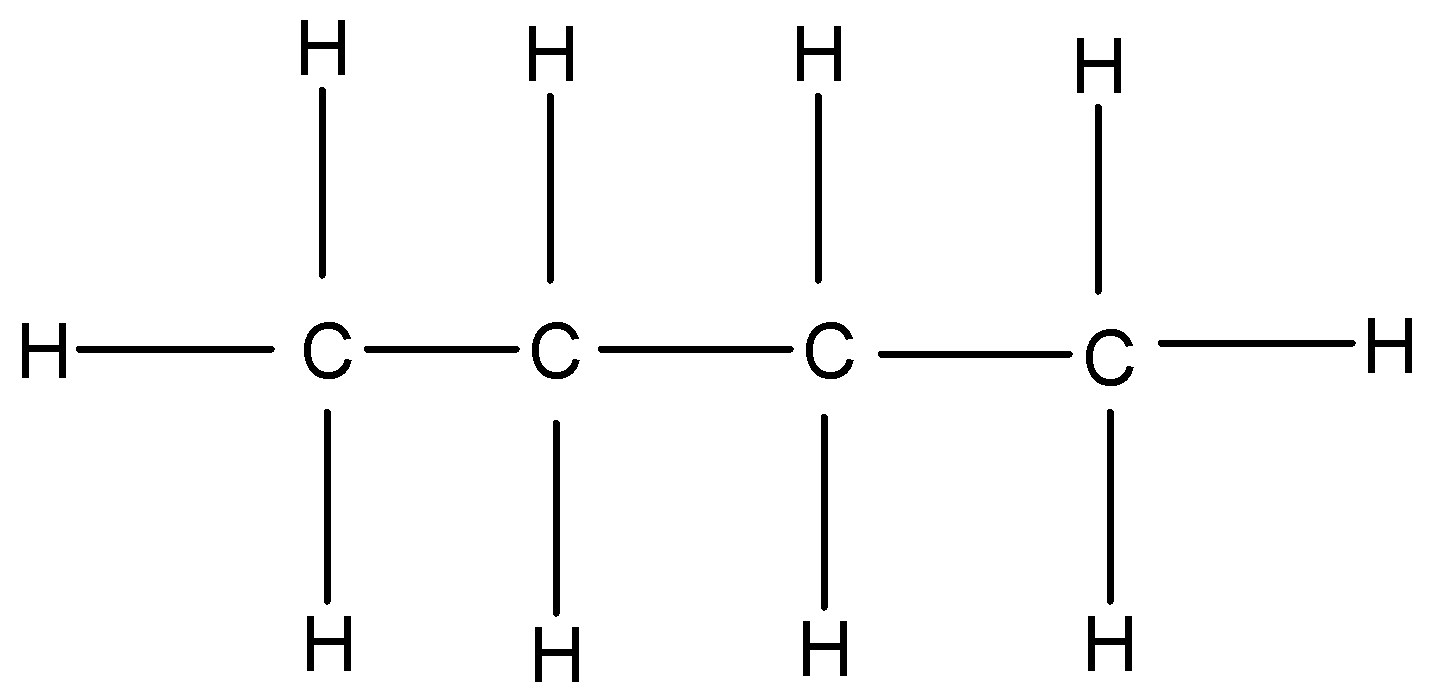
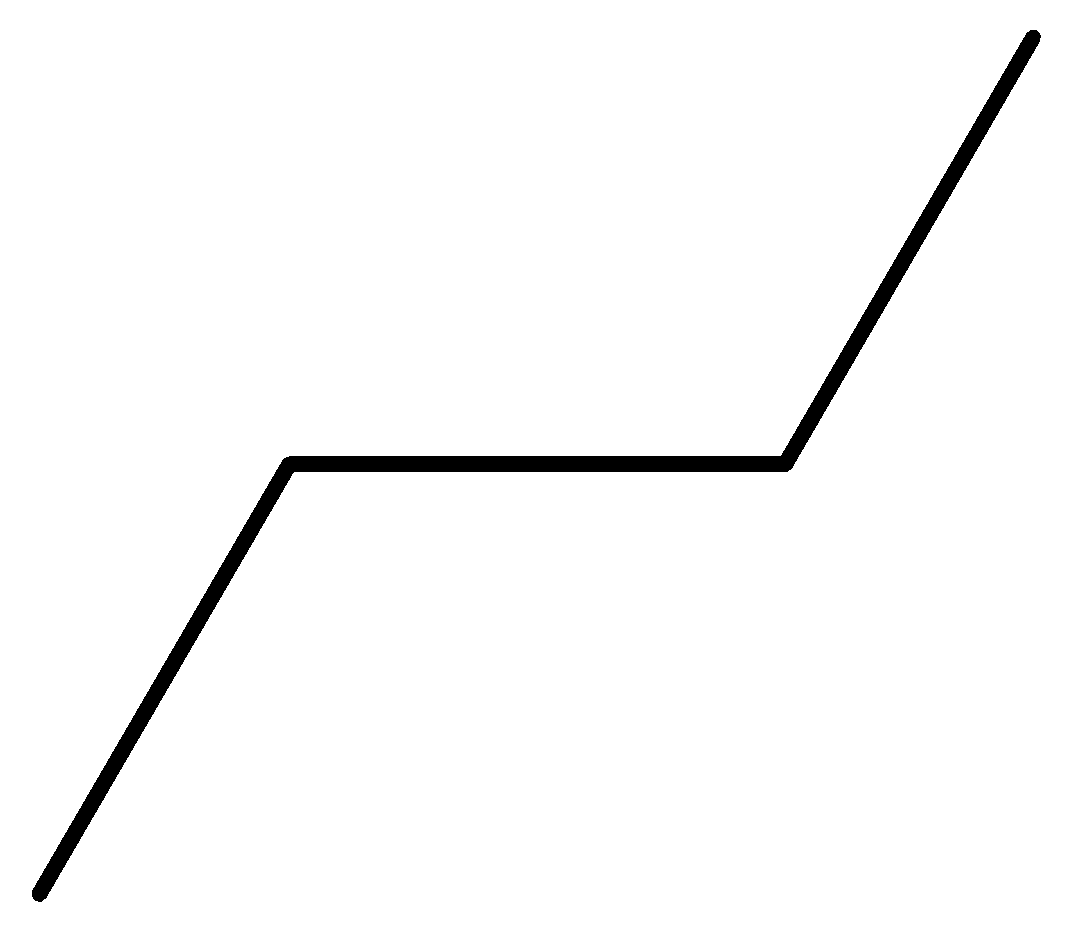
Figure: Structure formula of n-butane
Iso-butane: The other isomer is iso-butene or 2-methyl propane or i-butane. Iso-butane has a propane parent chain with a methyl group −CH3 attached to the second carbon of the chain. That is why its IUPAC name is 2-methyl propane.
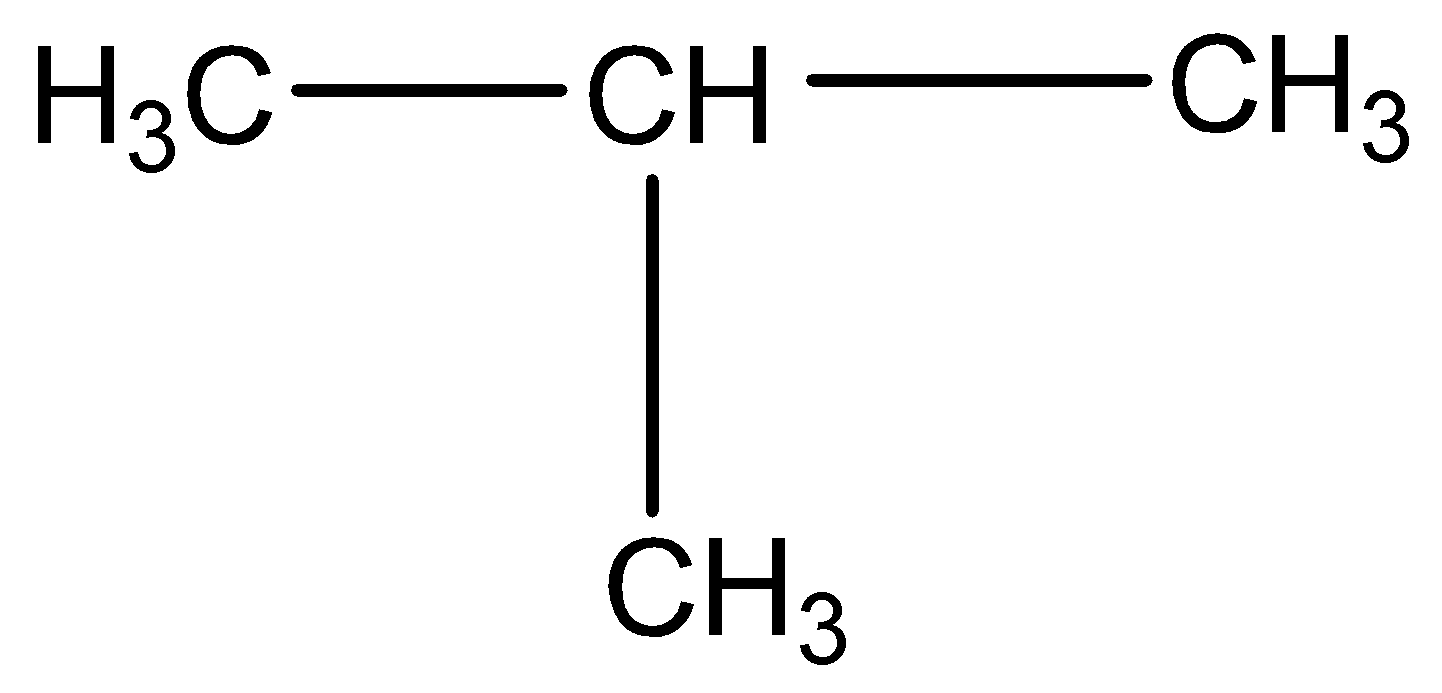
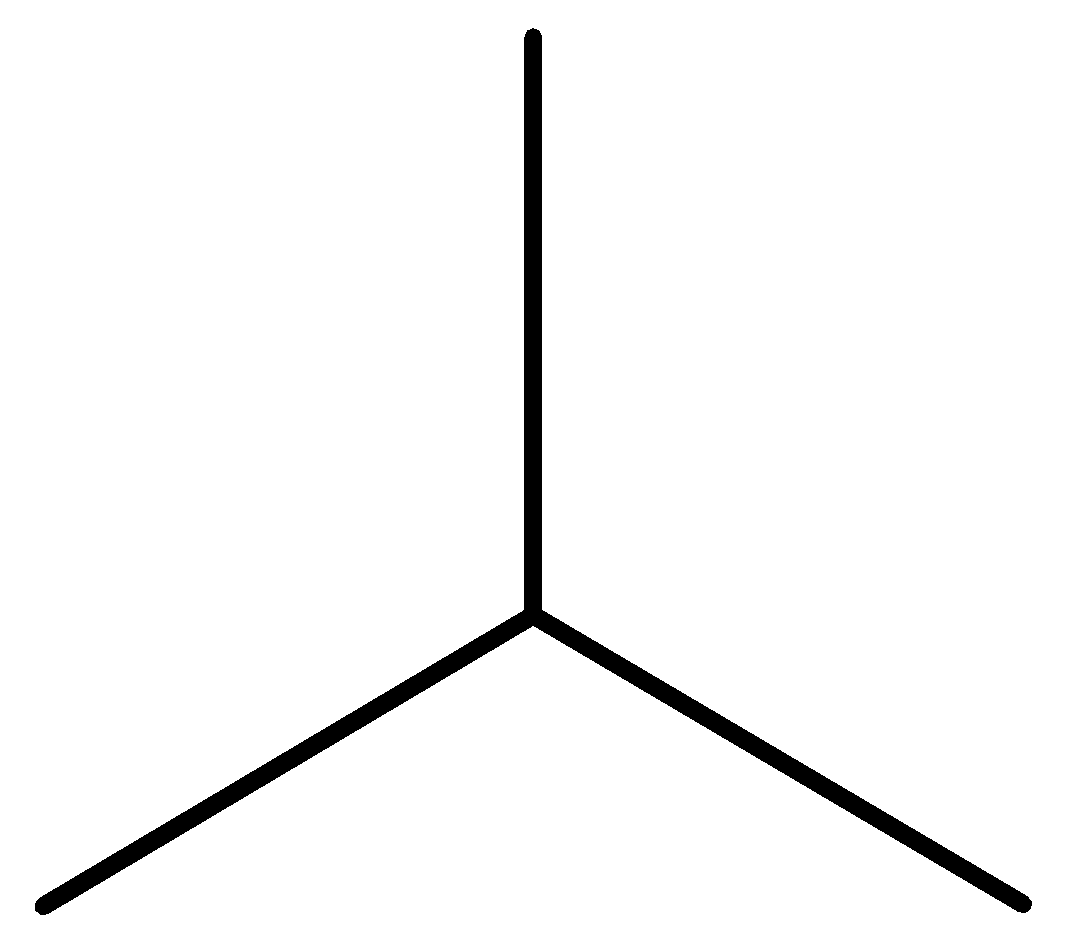
Figure: Structural formula of iso-butane
Note:
The simplest hydrocarbons with all C−C bonds are alkanes. That is the reason they are called saturated hydrocarbons. The general formula for alkanes is CnH2n+2 . In alkanes. all carbon atoms tend to complete their tetra valency by bonding with the same or different atoms and all the carbon atoms form single covalent bonds with other carbon atoms. The parent chain can be branched or unbranched and on the basis of that chemical and physical properties change. Alkanes are comparatively less reactive than hydrocarbons like alkenes, alkynes etc. because all carbon atoms are bonded with single covalent bonds in alkanes which are strong and less reactive in comparison to double or triple covalent bonds of alkenes and alkynes respectively.
