Question
Chemistry Question on Coordination Compounds
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
(i)K[Cr(H2O)2(C2O4)2].3H2O
(ii)[Co(NH3)5Cl]Cl2
(iii)CrCl3(py)3
(iv)Cs[FeCl4]
(v)K4[Mn(CN)6]
(i) Potassium diaquadioxalatochromate (III) trihydrate.
Oxidation state of chromium=3
Electronic configuration: 3d3:t2g3
Coordination number = 6 Shape: octahedral
Stereochemistry:
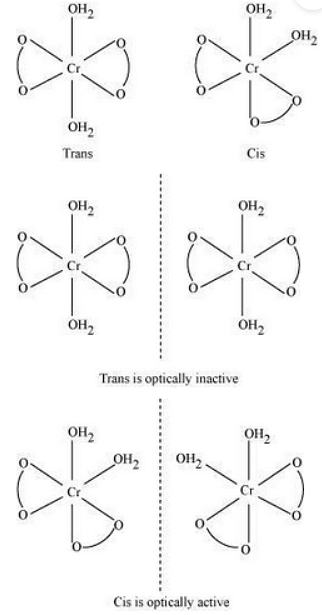
Magnetic moment, A~ZˇA^41=n(n+2)
=3(3+2)
=15
A~¢E¨†A^¼4BM
(ii)[Co(NH3)5Cl]Cl2
IUPAC name: Pentaamminechloridocobalt(III) chloride
Oxidation state of Co=+3
Coordination number=6 Shape: octahedral.
Electronic configuration: d6:t2g6.
Stereochemistry:

Magnetic Moment=0
(iii)CrCl3(py)3
IUPAC name: Trichloridotripyridinechromium (III)
Oxidation state of chromium = +3
Electronic configuration for d3=t2g3
Coordination number = 6
Shape: octahedral.
Stereochemistry:

Both isomers are optically active.
Therefore, a total of 4 isomers exist.
Magnetic moment,A~ZˇA^41=n(n+2)
=3(3+2)
=15
∼ 4BM
(iv)Cs[FeCl4]
IUPAC name: Caesium tetrachloroferrate (III)
Oxidation state ofFe=+3
Electronic configuration of d6=eg2t2g3
Coordination number = 4 Shape: tetrahedral
Stereochemistry: optically inactive Magnetic moment: A~ZˇA^41=n(n+2)
=5(5+2)
=35
~6BM
(v)K4[Mn(CN)6] Potassium hexacyanomanganate(II)
Oxidation state of manganese=+2
Electronic configuration: d5+:t2g5
Coordination number = 6 Shape: octahedral.
Streochemistry: optically inactive
Magnetic moment,A~ZˇA^41=n(n+2)
=1(1+2)
=3
=1.732BM
