Question
Question: : Write down the formulae of sodium sulfide....
: Write down the formulae of sodium sulfide.
Solution
We know that, joining of atoms of different elements in definite proportions forms compounds. Some examples of compounds are water (H2O), ammonia (NH3). In water, hydrogen and oxygen are present in the fixed ratio of 1:8.
Complete step by step answer:
Let's discuss the writing formula of a compound. Chemical formula of a compound shows the composition of the compound using symbols of combining atoms. To write the formula, we need the valency of the combining elements.
Let’s discuss valency of an element. The capacity of combination of elements is termed as valency. In simple words valency gives the idea how an atom of an element combines with atoms of another element. Valency of sodium is +1, magnesium is +2 and aluminium is +3 etc.
To write the chemical formula of a compound, first we have to write the chemical symbols of the elements along with the valency then, we have to crossover the valencies of the combining elements.
Let’s come to question. We have to write the formula of sodium sulfide. So, the two elements present in the compound are sodium and sulphur. The valency of sodium is +1 and sulphur is -2.
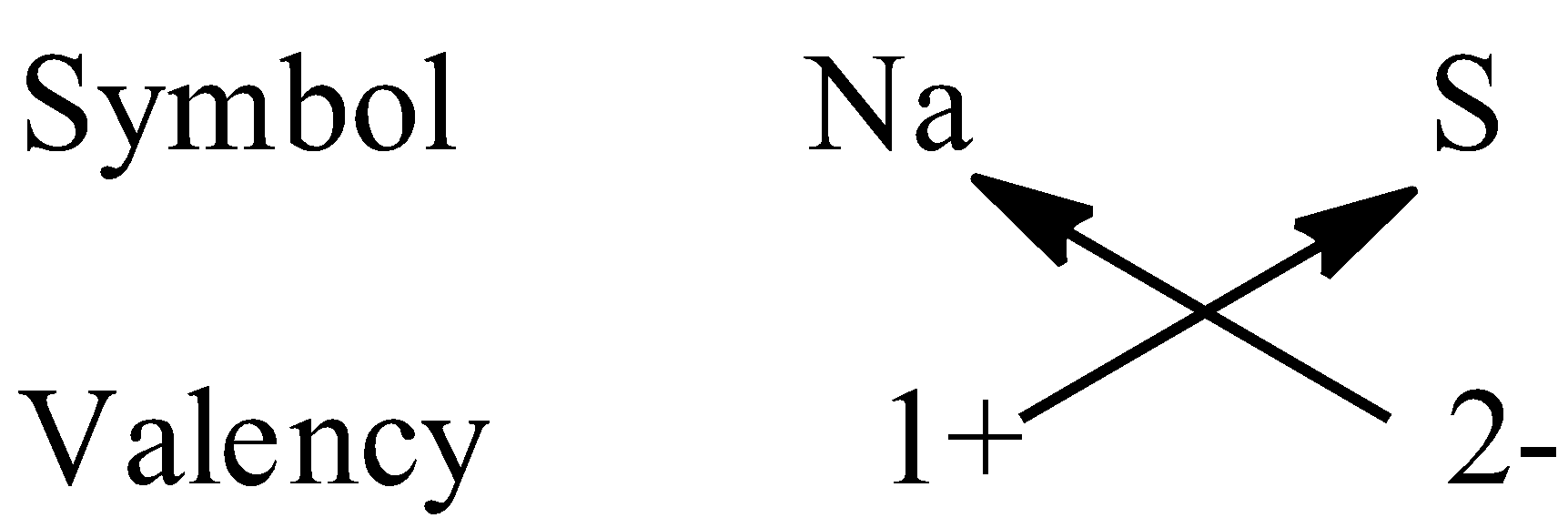
So, the formula of sodium sulphide is Na2S.
Note:
There are some rules to be followed while writing chemical formulas of compounds
- In compounds having a metal and non-metal, the symbol of metal is to be written first. For example in the compound calcium oxide (CaO), calcium is written first as it is a metal.
- The valencies on the ion must balance
- In case of compounds having polyatomic ions such as hydroxide ion (OH), polyatomic ion is to be enclosed within brackets.
