Question
Question: Write dash formulae for the following bond line formula. A.
B.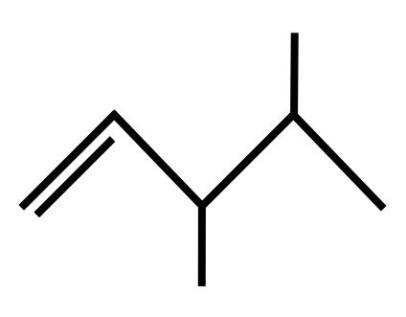
C.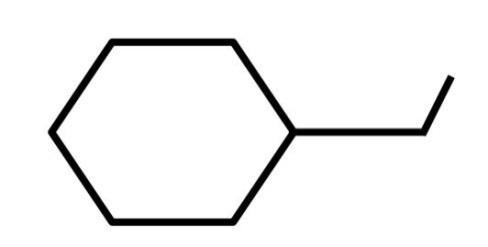
D.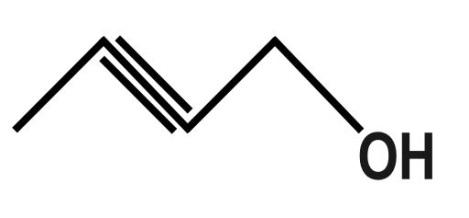
Solution
We know that the chemical formula in which there is no presence of sigma or pi bonds is called condensed formula. The bond line formula is nothing but we have to represent the total structure of the compound without using the carbon symbol. The concept of bond line formulas is to be used in this question. In the given compound, there are carbons that have methyl and ethyl groups attached to the sides and these contribute to increasing the molar mass of the compound.
Complete answer: As we know that the concept of bond line formula and dash formal will be used;
1.Bond Line Structure: This is a structure representation of organic compounds. Carbon and hydrogen atoms are not indicated, but only the bonds between carbons atoms are indicated in the form of lines.
2.Dash Formula: The Dash formula is a representation of all the bonds of the compound using dash that specifies the bond between the two atoms.
In order to answer our question, we need to learn about the bond line formula for organic compounds. Now, carbon has a valency of four. That means one carbon can attach with itself four other groups or atoms, so that its octet gets filled. This results in the cast range of compounds in the field of organic chemistry. Now, due to the excessive number of compounds, there are some compounds which are very complex or large and it is very time consuming to draw structures of them. Moreover, they will look very messy on paper.
So, bond line formulas are preferred. Bond line structures are very simple to draw and understand the structure of very large compounds of organic chemistry. These days, the two dimensional formulae of the organic compounds are also shown with the help of bond line notations in which the bonds are shown with the help of lines only. For example, a single line (−) represents a single C to C bond ; a double line (=) indicates a double C to C bond while a C to C triple bond is shown by a triple line (≡).
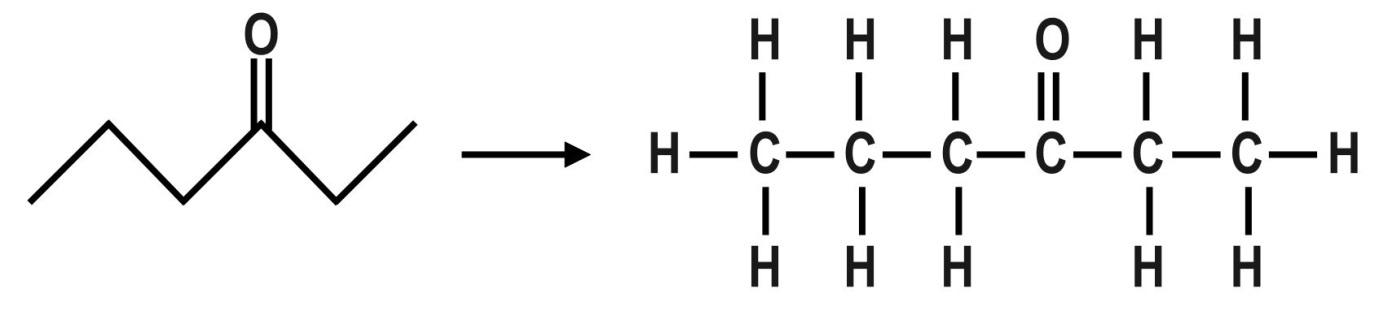
A.The first organic compound is Hex−3−one or 3−Hexanone. We can also say Ethyl propyl ketone. The molecular formula of this compound is: C6H12O The dash formula of this compound is:
B.The second organic compound is: 1−ethylhexane. The molecular formula of this compound is: C8H16. The dash formula of this compound is:
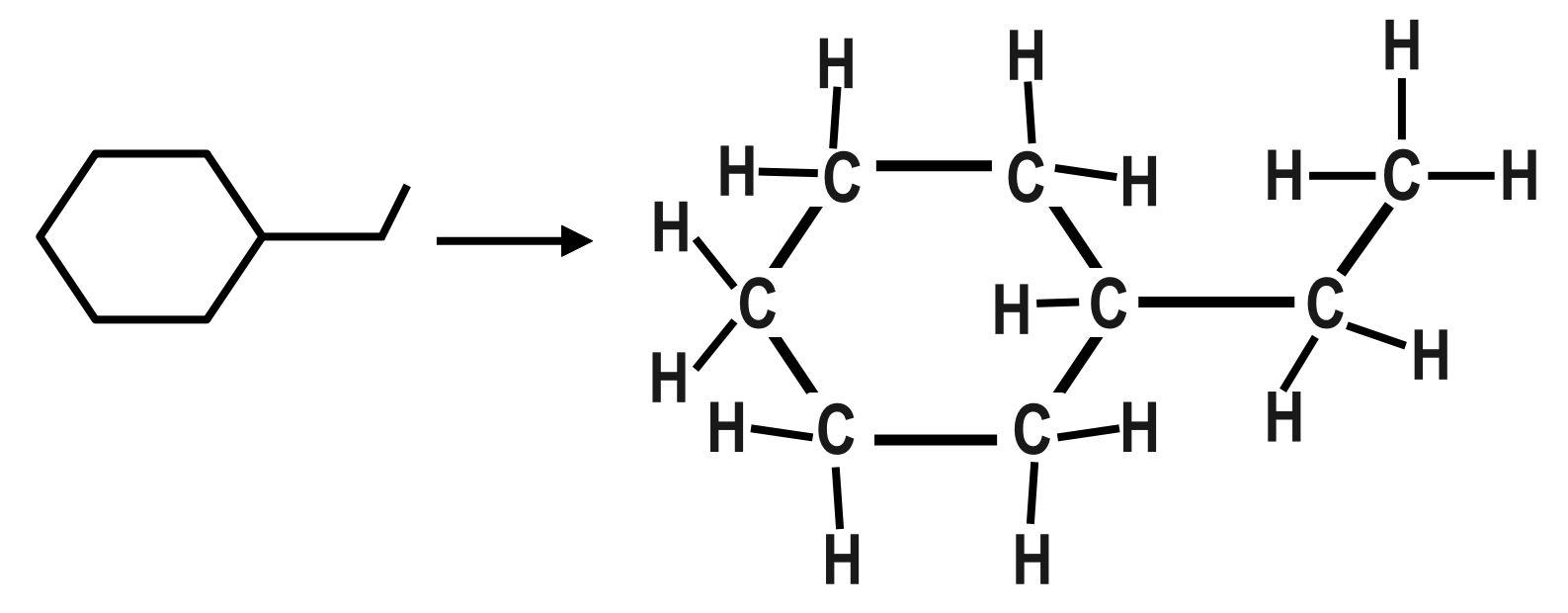
C.The third organic compound is: 3,4−dimethylpent−1−ene or The molecular formula of this compound is: C6H14. The Dash formula for this compound is:
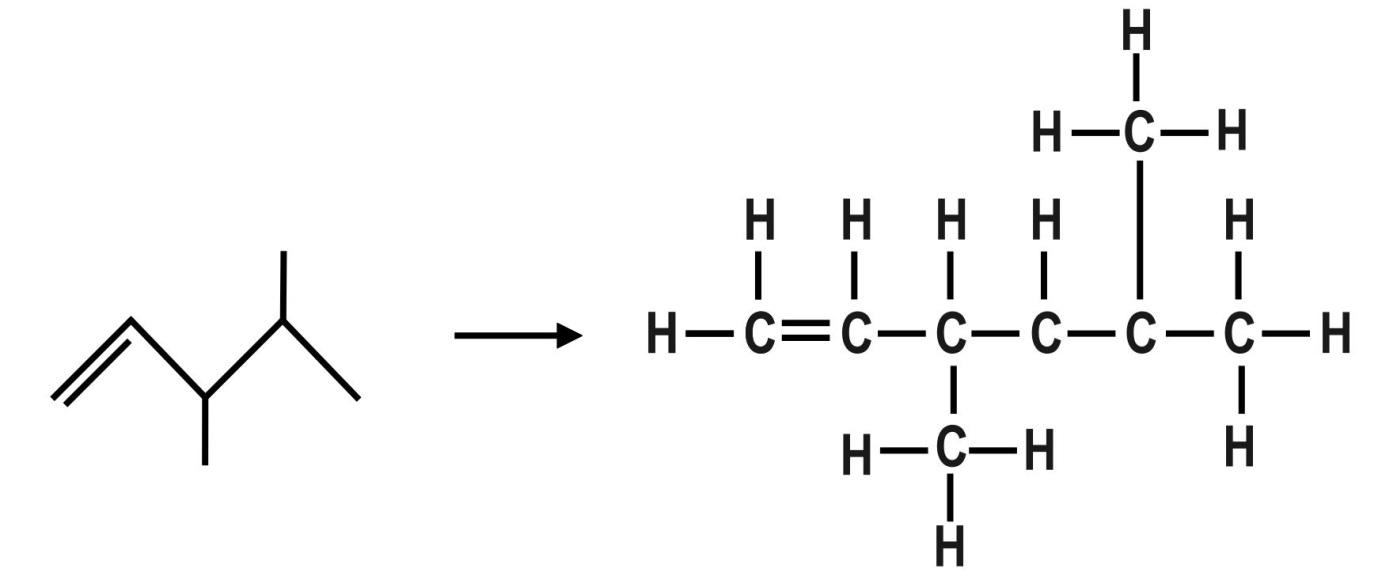
D.The fourth organic compound is: 2−Butyn−1−olor 2−Butynol. The molecular formula of this compound is: C4H6O. The dash formula of this compound is:
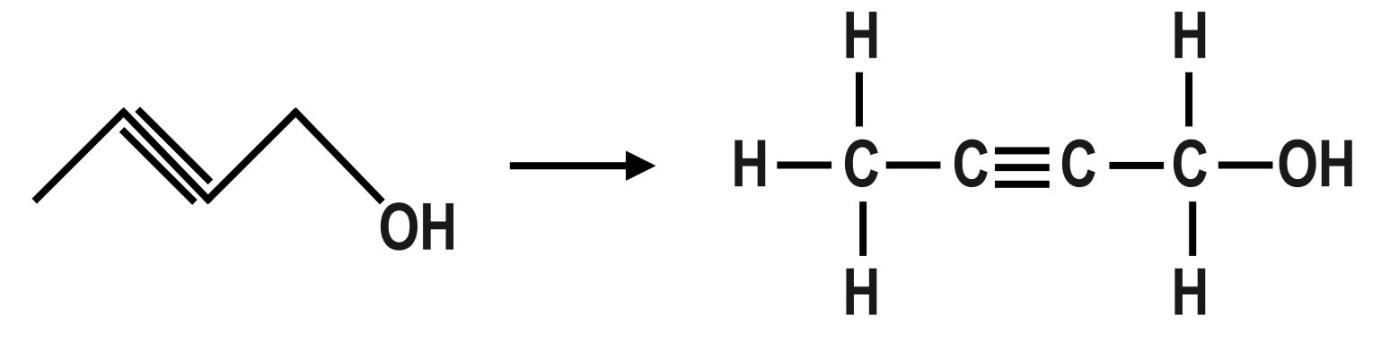
Note:
Remember that the organic compound can be depicted in many ways like complete structure which is also known as dash formula, condensed structure which we say as molecular formula and bond line structure. The bond line formula is for drawing 2D organic structures. It doesn't give us any idea about whether the bond is directed inside the plane or outside the plane. However, bond line formulas can give us an idea about steric hindrance.
