Question
Question: Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber pr...
Write chemical reactions for different steps in the manufacture of sulphuric acid by lead chamber process. Draw the structure of phosphorus pentachloride.
Solution
Chamber process is a method of producing sulphuric acid by oxidizing sulphur dioxide with moist air and the catalysts for the reaction is gaseous nitrogen oxide. The reactions take place in a series of large box-like chambers of lead sheet. Phosphorus has five electrons in its outermost shell and can form a single bond with five chlorine atoms each.
Complete step by step answer:
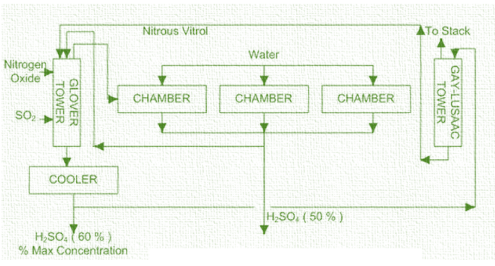
-The lead chamber process has three stages; Glover tower, lead chambers and Guy-Lussac Tower. The process begins with hot sulphur dioxide gas which enters the bottom of a reactor, Glover tower where it is washed with nitrous vitriol i.e. sulfuric acid with nitric oxide, NO, and nitrogen dioxide dissolved in it and mixed with nitric oxide and nitrogen dioxide gases.
-The Glover tower has two functions: concentration of the chamber acid and stripping of nitrogen oxides from the liquid to the gas. Concentration of the chamber acid is attained by the hot gases entering the tower which vaporise water from the acid. Some of the sulphur dioxide is oxidized to sulphur trioxide and dissolved in the acid wash to produce Glover acid. -The dissolved nitrogen oxides are stripped from the acid and carried out of the Glover tower into the lead chambers.
-From the Glover tower a mixture of gases is transferred to a lead-lined chamber where it reacts with more water. The chamber is a large, box-like room. Sulphuric acid is formed by a complex series of reactions i.e. it condenses on the walls and collects on the floor of the chamber. There may be from three to twelve chambers in a series and the gases pass through each chamber in succession. The acid produced in the chambers, often called chamber acid or fertilizer acid, contains 62% to 68% of sulphuric acid. The reactions taking place in the lead chamber are:
Overall reaction is SO2+21O2+H2O→H2SO4
After that, gases are passed into a reactor called the Gay-Lussac tower where they are washed with cooled concentrated acid from the Glover tower and the nitrogen oxides and unreacted sulphur dioxide dissolve in the acid to form the nitrous vitriol which is used in the Glover tower. The waste gases are usually discharged into the atmosphere. Product acid is drawn from the cooled acid stream circulated from the Glover tower to the Guy-Lussac tower.
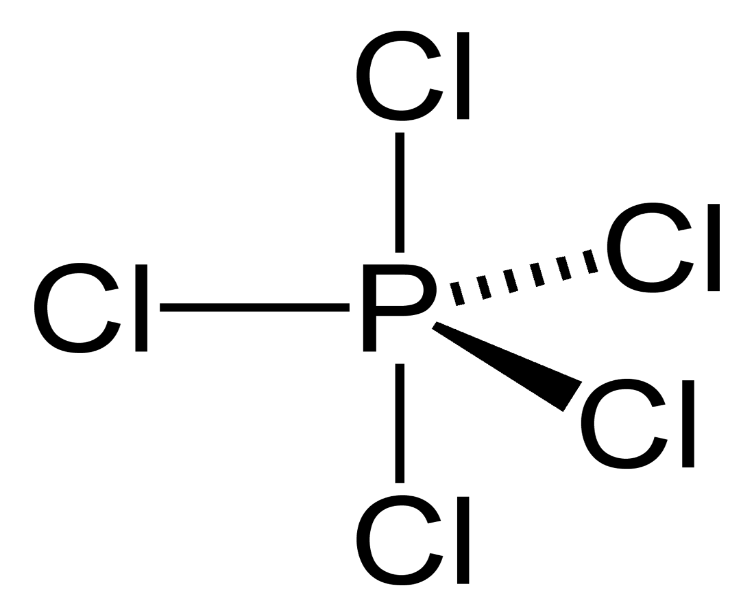
Phosphorus atoms belong to the nitrogen family and have five valence electrons. Five chlorine atoms bind to the phosphorus atom by five single bonds. Therefore, the structure of phosphorus pentachloride is trigonal pyramidal.
Note:
Sulphuric acid can also be manufactured by the Contact process. It makes sulphur dioxide and converts it into sulphur trioxide which is further converted into concentrated sulphuric acid in the presence of a catalyst, vanadium pentoxide. The product is fuming sulphuric acid or oleum.
