Question
Question: Write chemical equations for the following conversion: Aniline to benzyl alcohol...
Write chemical equations for the following conversion:
Aniline to benzyl alcohol
Solution
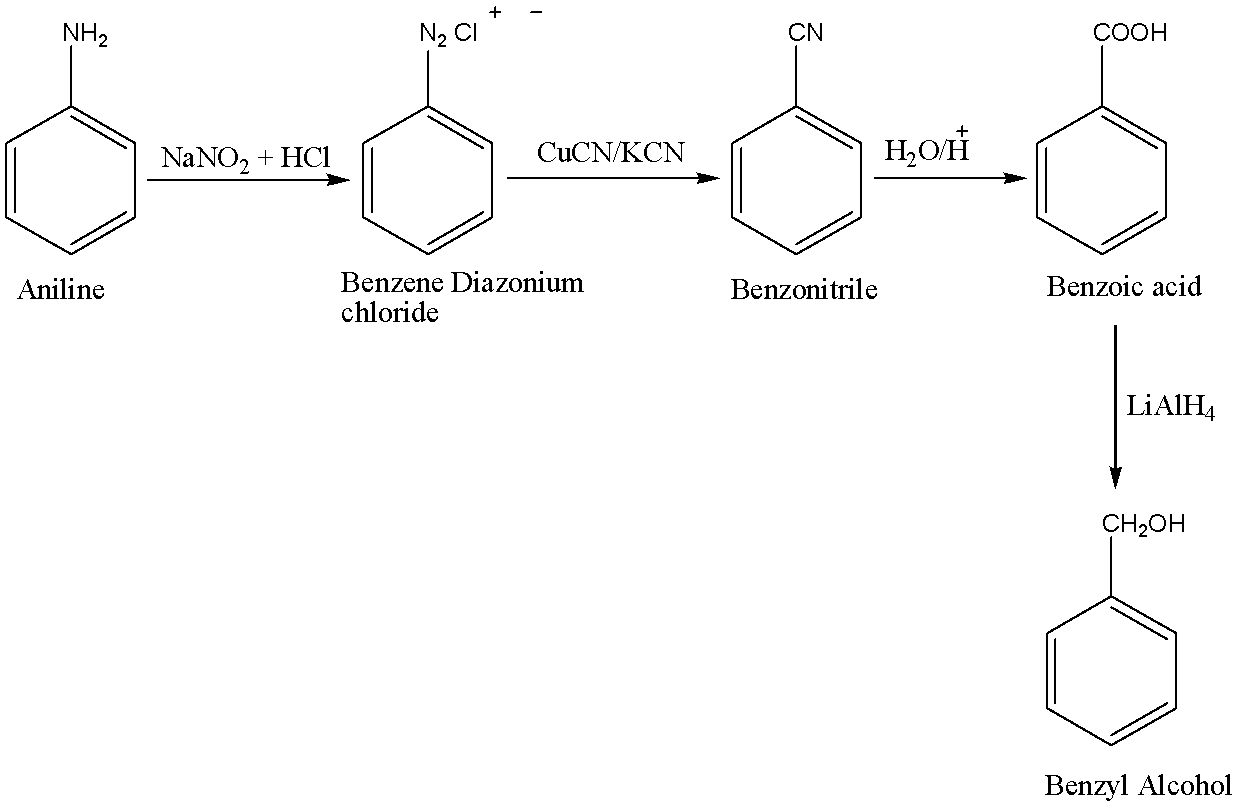
We know that in the presence of excess acid, n-nitrosamine can be converted into a diazohydroxide by protonation and subsequent deprotonation. Diazohydroxide is now protonated and water is removed from the compound to give the required aryl diazonium ion which can easily be converted into a diazonium salt and Sandmeyer reaction is a type of substitution reaction that is widely used in the production of aryl halides from aryl diazonium salts.
Complete answer:
First, the nitrosation of aniline with nitrous acid which is generated in situ from sodium nitrite and a strong acid, such as hydrochloric acid, sulphuric acid, or HBF4) takes place which further leads to diazonium salts, which can be isolated if the counter ion is non-nucleophilic. Diazonium salts are important intermediates for the preparation of halides in reactions like Sandmeyer Reaction, Schiemann Reaction, and azo compounds. Diazonium salts can react as pseudohalide-type electrophiles. So, in this case Benzene Diazonium chloride is formed. The reaction is carried out at temperatures ranging between 273−2780C.
Secondly, in the Sandmeyer reaction, the amino group that is attached to an aromatic ring is converted into a diazonium salt that can be transformed into various functional groups and in this case benzonitrile is formed.
Thirdly, Benzonitrile is converted to benzoic acid by basic hydrolysis. When benzonitrile is heated with aqueous sodium hydroxide solution, it liberates ammonia gas and converts to sodium benzoate which on acidification gives benzoic acid. Benzoic acid contains a carboxylic acid functional group.
In the last process, benzoic acid on reduction with the help of LiAlH4 gives benzyl alcohol.
The chemical equations for the following conversion is shown below:
Note:
It should be noted that we should not get confused between the benzyl, benzal and benzo prefixes. When we say benzyl, it means that the carbon that is attached to the benzene ring has two hydrogen atoms. While in the case of benzal, the carbon carries one hydrogen atom and in the case of benzo, there is no hydrogen atom attached to the carbon that is directly linked or bonded to the benzene ring.
