Question
Question: Write at least 5 compounds with the structural formula of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\tex...
Write at least 5 compounds with the structural formula of C3H6O and identify the functional groups.
Solution
Hint: Organic compounds with similar molecular formula but different structural formula and presence of different functional groups are called Functional isomers of each other.
Complete step by step answer:__
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures.
Different structures are possible of a compound having formula C3H6O. These are –
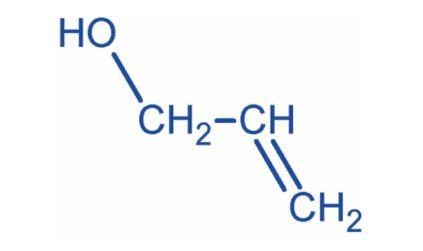
Prop-2-ene-1-ol
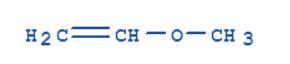
Methoxyethene
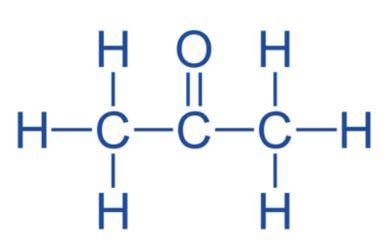
Propanone
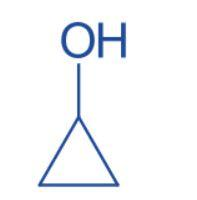
Cyclopropanol
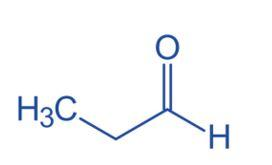
Propanal
Additional information:
When the atoms and the functional groups are joined together in different ways but the molecular formula remains the same, the type of isomerism is called Structural isomerism. There are a few types of structural isomerism:
(I) Chain Isomerism
When the carbon molecules in the chain of an organic molecule are relocated but the molecular formulas remain the same, Chain isomerism is confirmed. This alters the chain of the organic compound. The number of possible structural isomers increases greatly with the number of available atoms.
(II) Position isomerism
When compounds having the same molecular formula, same structural formula but the position of functional groups between two identical organic compounds is different, the compounds are said to show positional isomerism.
(III) Functional isomerism
Organic compounds which have the same molecular formula but the functional group present is different are called functional isomers of each other and the phenomenon is called functional isomerism.
Note: In the above given example, all compounds have same molecular formula i.e. C3H6O but have different functional groups and hence are said to be functional isomers of each other. Total 11 isomers are possible with the molecular formula C3H6O.
