Question
Question: Write any ten examples of IUPAC nomenclature of alcohol....
Write any ten examples of IUPAC nomenclature of alcohol.
Solution
As we have learnt that alcohols are the compounds possessing hydroxyl functional groups attached with an aliphatic carbon atom. Alcoholic compounds possess sp3 hybridised oxygen as central atom.
Complete step by step solution: Alcohols may be classified as mono, di, tri-or polyhydric compounds depending upon the number of hydroxyl groups respectively in their structures. The IUPAC name of an alcohol is derived from the name of an alkane and adding the suffix “ol” in place of ‘e’ in alkane. For example:
Methane+ ol = methanol (CH3OH)
Ethane + ol = ethanol (C2H5OH)
Propane + ol = propanol (C3H7OH)
Butane + ol = butanol (C4H9OH)
Pentane + ol = pentanol (C5H11OH)
Vinylic alcohol = CH2=CH−OH
For naming of polyhydric alcohols, the ‘e’ of alkane is retained and the ending “ol” is added. The number of −OH groups is indicated by adding the multiplicative prefix di, tri, etc. before ‘ol’. Let us take an example:
HO−CH2−CH2−OH the name of this alcoholic compound is ethane−1,2−diol.
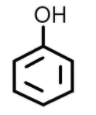
Similarly benzylic alcohols can also be named using the suffix ‘ol’. For example: Phenol (C6H5OH)
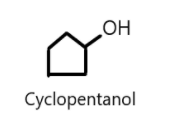
Cyclic alcohols can also be named using prefix cyclo and suffix ‘ol’ considering the hydroxyl group is attached to the first carbon atom of the ring. For example: Cyclopentanol (C5H8OH)
We can also name the branched compounds by adding a number of branches and suffix ‘ol’ at the end. For example: 2−Methylpropan−2−ol (CH3)3−C−OH
so we can easily give the name of ten alcoholic compounds as:
Methanol, Ethanol, Propanol, Butanol, Pentanol, Phenol, Cyclopentaol, 2−Methylpropan−2−ol,ethane−1,2−dioland vinylic alcohol.
Note: Some basic rules for naming alcohol includes the longest continuous chain containing −OH group is the parent chain and the parent compound, alkane is replaced by the suffix ‘ol’ at ‘e’ in alkane and in cyclic alcohols the carbon atom bearing the −OH is designated as C1 but the name does not include 1.
