Question
Question: Write any four differences between lithium and other alkali metals....
Write any four differences between lithium and other alkali metals.
Solution
Hint: Alkali metals are the chemical elements in group 1 of the periodic table of elements except for hydrogen. Thus, the members of this group that fall into this category are Lithium, Sodium, Potassium, Rubidium, Caesium and Francium. The reason why we name them as alkali metals is that they form alkali compounds.
Complete step by step answer:
The key difference between Lithium and other alkali metals that we can tell is that the lithium is the only alkali metal that can react with nitrogen whereas the other alkali metals cannot undergo any reaction with nitrogen. Moreover, lithium cannot form an anion while other alkali metals can form anions. Melting and boiling point is higher than other alkali metals .
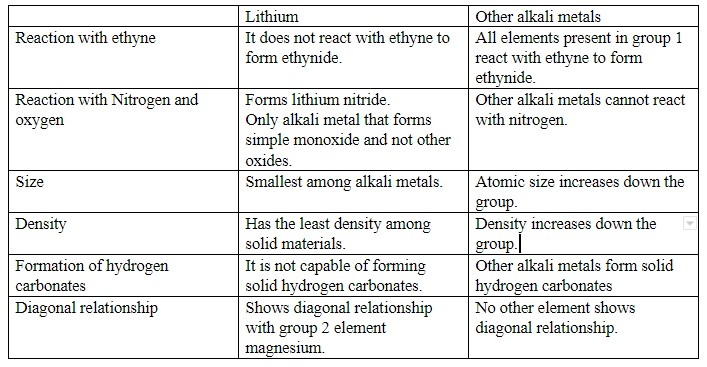
Rest of the differences are explained in below table
Note:
Lithium has very low affinities for electrons, moreover requires relatively little energy to lose an electron. Also due to their small size, they are unable to accommodate more electrons as adding an electron can increase the electron-electron repulsions within the atom .
