Question
Question: Write an equation to show that 2,3-dimethylbutane may be prepared from each of the following compoun...
Write an equation to show that 2,3-dimethylbutane may be prepared from each of the following compounds. (i) an alkene (ii) a grignard reagent (iii) a haloalkane (iv) a sodium alkanoate?
Solution
2,3-dimethylbutane is generally a form of alkane which consist of long chain of 4 carbon atoms known as the name butane and branching of two methyl groups are present at position 2 and 3 which termed as dimethyl.
Complete answer:
(i) An alkene: Alkene contains unsaturation in it i.e. double bond is present in alkenes. The equation can be written as:
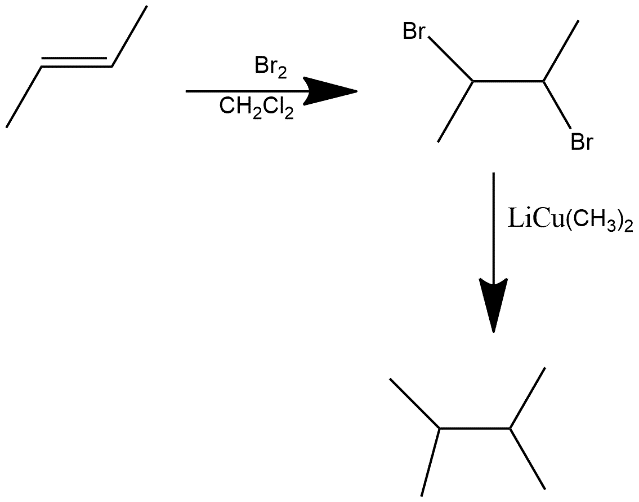
In first step bromination is done and then two equivalents of LiCu(CH3)2gives us the 2,3-dimethylbutane.
(ii) a grignard reagent: This can be shown as follows:
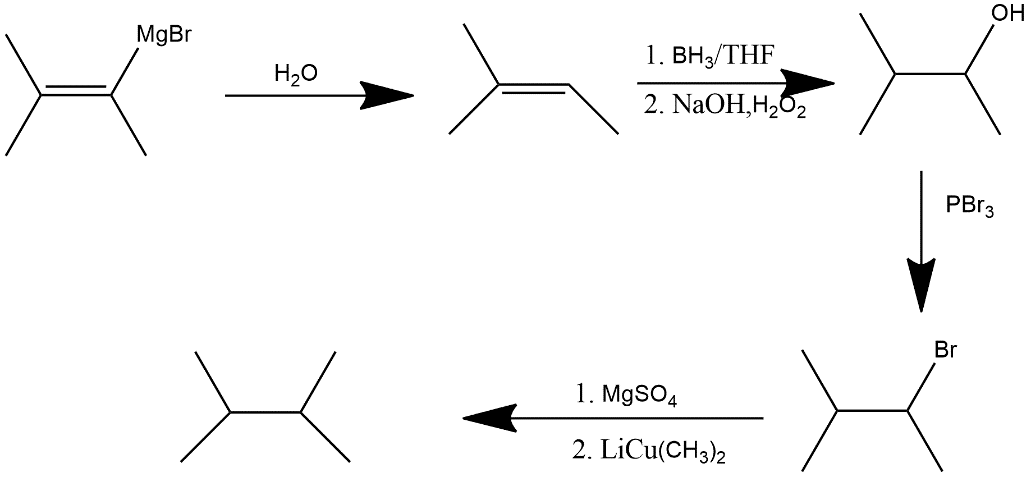
Water gets rid by the magnesium bromide substituent and substitutes it with a hydrogen, then Hydroboration adds a hydroxide on the carbon where the magnesium bromide is added according to anti-Markovnikov rule and PBr3 substitutes the hydroxide with a bromide group thenMgSO4 acts as a drying agent to clear the reaction vessel of any water remaining from steps 1 and 2, LiCu(CH3)2 substitutes a methyl group in place of the bromide group.
(iii) a haloalkane:
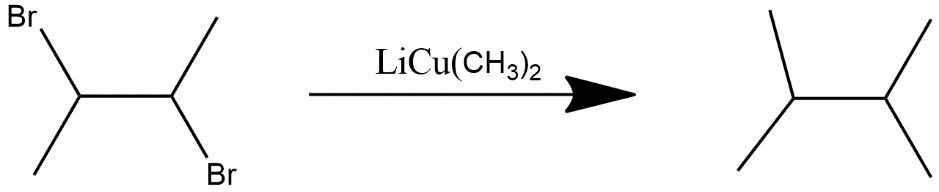
This is the same as alkene.
(iv) a sodium alkanoate
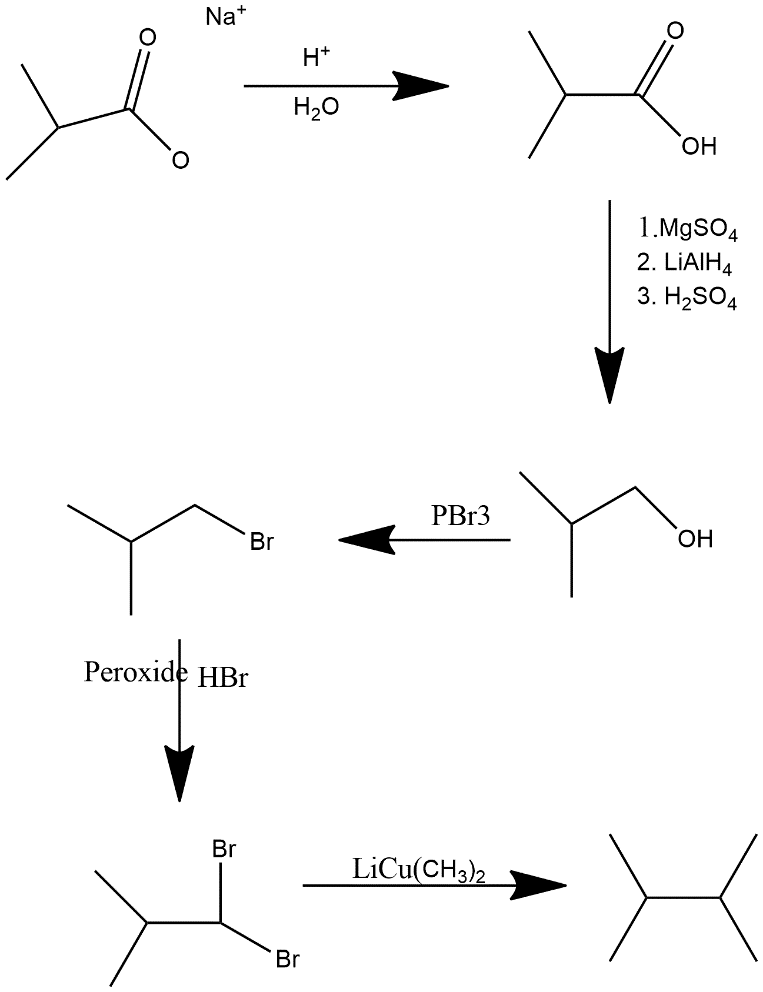
In first step strong acid protonated the alkanoate and make it carboxylic acid then MgSO4 MgSO4, LiAlH4 and dilute sulfuric acid acts as reducing agent turns acid in alcoholic group after that same step occurs as in equation i.
Note:
Alkyl halides are also known by the name halo alkanes or halogen alkanes. These are chemical compounds which are derived from alkanes which contains one or more halogen atoms. These are also known as these are a subset of the general class of halocarbons.
