Question
Question: Write a brief note on hyperconjugation and give all hyper conjugated structures of propene....
Write a brief note on hyperconjugation and give all hyper conjugated structures of propene.
Solution
Hint- Proceed the solution of this question using the concept of hyper-conjugation, which is a special case of resonance that involves delocalisation of σ electrons of C−H bond of any alkyl group. Hence, in the same way show the delocalisation of σ electrons of C−H bond in propene structure.
Complete answer:
Hyper-conjugation is the interaction of the electrons in a σ bond with an adjacent empty or partially filled non-bonding p-orbital, antibonding σ or π orbital or filled π orbital to give an extended molecular orbital that increases the stability of the system.
In case of propene, hyper conjugation arise due to partial overlap of sp3 sigma bond orbital and the empty p-orbital of an adjacent c−atom.
Here one of the C-atom C−H bonds of −CH3 group can lie in the plane of pi-bond orbital, hence partial overlapping.
Hyper conjugation structures of propene are given below-
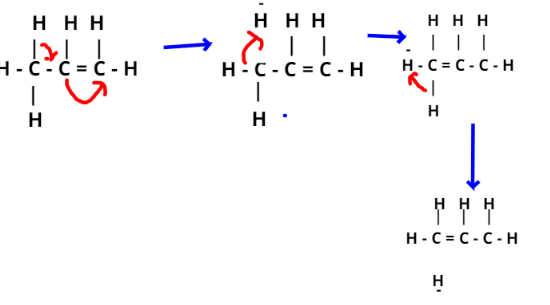
Note- In this particular question, we should know that Hyperconjugation is also known as no bond resonance. You may note that resonance involves delocalisation of π electrons but it’s the σ electrons that are delocalised in hyper conjugation. The normal carbon-carbon single bond length is 1.54 A0. However, in some compounds such as propene, it is a little shorter because of presence of some double bond character in C−C single bond due to hyper conjugation
