Question
Question: With which of the following does trichloroethene react to give a chiral product? 
(a) Br2
(b) HCl
(c) NaCN(aq)
(d) NaOH(aq)
Solution
A chiral compound is a compound which contains a sp3 hybridized carbon atom which is attached to four different carbon atoms. Such a compound shows optical isomerism.
Complete step by step answer:
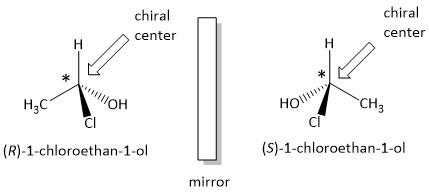
A molecule is referred to as chiral if it is not superimposable on its mirror image. For example a pair of hands is also non superimposable on one another and is chiral. The molecule which exhibits such kind of property is said to possess geometrical isomerism.
A chiral molecule is said to show stereoisomerism which have the same molecular formula, same bonded atoms in the same sequence but differ in three dimensional arrangements in space. Molecules generally show two kinds of stereoisomers, one in enantiomers and the other is diastereomers. Enantiomers are non-superimposable mirror images of one another but diastereomers are not mirror images.
For example the following molecule is a chiral molecule which is attached to four different substituents.
The given substrate is trichloroethene which is an alkene compound. Both the carbon atoms are bonded by double bonds and are sp2 hybridized. It is an electron rich molecule and undergoes an electrophilic addition reaction to produce sp3 hybridized carbon atoms. Let us determine which of the given reagents can undergo electrophilic addition reaction with alkene to produce chiral product.
(a) Br2. Molecular bromine is known as a good electrophile and undergoes additional reaction with electron rich substrates. Thus the product formed from the addition of bromine with the substrate trichloroethene is:
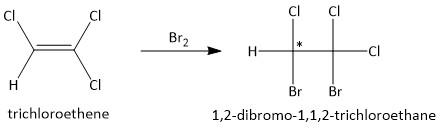
One of the two sp3 hybridized carbon atoms is attached to two four different substituents and is a chiral product.
(b) HCl. The addition of hydrochloric acid to alkene results in the formation of two compounds. The hydrogen and the chlorine attach itself to either of the two double bonded carbon atoms. Thus the two products are:
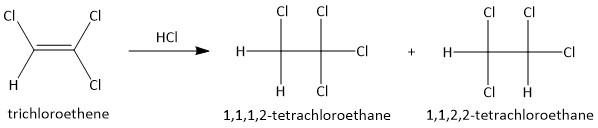
Here none of the products has an sp3 hybridized carbon atom which is attached to four different substituents. Hence none of the products are chiral.
(c) NaCN(aq). It does take part in electrophilic addition reactions. It rather undergoes substitution reaction replacing any of the chlorine atoms from the molecule. Thus no sp3 hybridized compound is generated and the product is not chiral.
(d) NaOH(aq). Here also no electrophilic addition reaction occurs. It rather undergoes substitution reaction replacing any of the chlorine atoms from the molecule. So no sp3 hybridized compound is generated and the product is not chiral.
Hence,option a is the correct answer.
Note: Chirality is the independent property of a molecule. The change of the plane of a polarized light in a polarimeter measures the amount of optical rotation of the chiral molecule.
