Question
Question: With the reference of the scheme given, which of the given statement (s) about T, U, V and W is corr...
With the reference of the scheme given, which of the given statement (s) about T, U, V and W is correct?
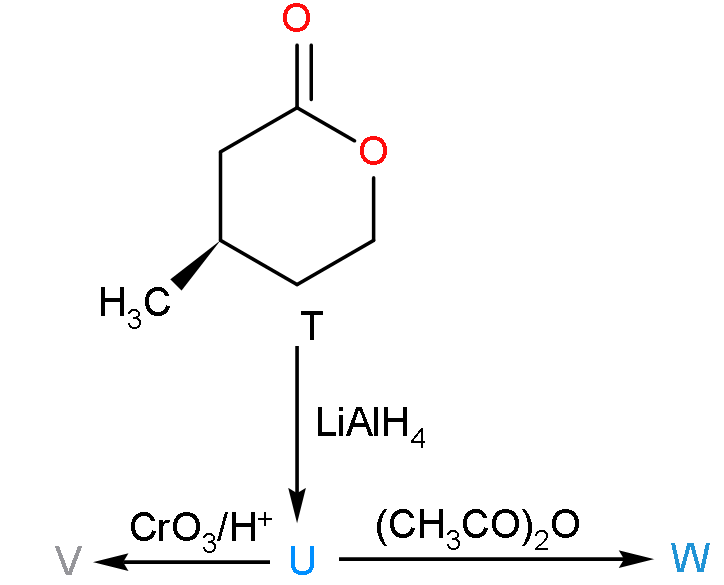
A. T is soluble in hot aqueous NaOH
B. U is optically active
C. Molecular formula of W is C10H18O4
D. V gives effervescence on treatment with NaHCO3
Solution
For this problem, firstly we have to write the product which will be formed when the compound T will react with Lithium aluminium hydroxide and then the further reaction to write the products V and W so that we can determine all the options.
Complete step by step answer:
- In the given question, we have to explain that among the given options which option is correct or not.
- Now, firstly the compound T will reduce when it will react with lithium aluminium hydroxide because it is a reducing agent so the product formed will be:
- Now, here the compound T when placed in the alkaline medium of sodium hydroxide then it will form a salt complex due to which it will be soluble.
- So, we can say that the compound T will be soluble in the hot aqueous solution of the sodium hydroxide.
- Now, when the compound U will react with acetic anhydride with highly acidic because it consists of two molecules of acetic acid.
- Then it will yield a compound as shown below:
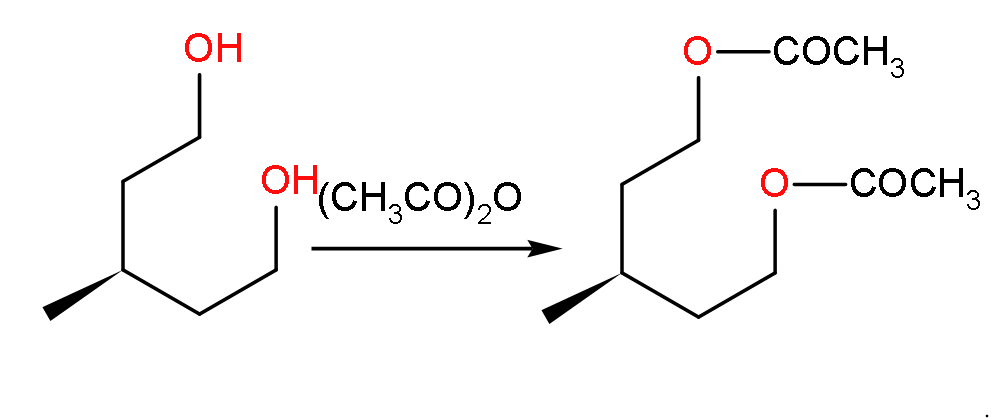
- So, here the product formed will have the molecular formula of C10H18O4 because we can see in the structure that there are 10 carbon atoms, 18 hydrogen atoms and 4 oxygen atoms.
- So, we can say the statement C is correct.
- Now, firstly we have to find out the product V which is formed when the molecule U will undergo oxidation due to the presence of CrO3/H+.
- So the product formed will be:
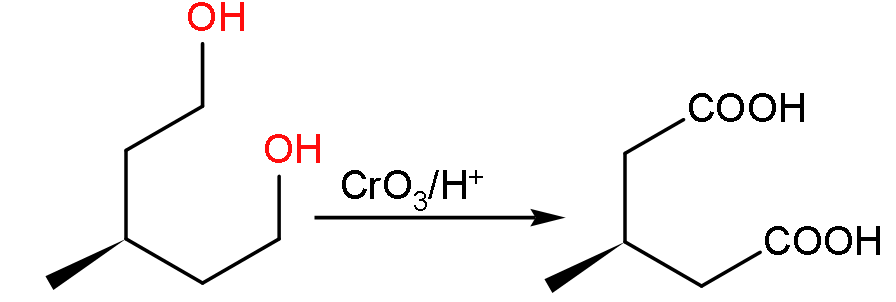
- Now, the molecular formula of the compound V will be C6H10O4 so when it reacts with sodium carbonate it gives carbon dioxide gas.
- Due to the production of carbon dioxide gas it will show the effervescence as shown below:
C6H10O4 + NaHCO3 → CO2↑
So, the correct answer is “Option A, C and D”.
Note: In this problem, the compound U is not the optically active compound because there is no chiral carbon present. As we know that the chiral carbon is the carbon which is attached to the four different groups.
