Question
Question: With respect of chlorine, hydrogen will be: (A) Electropositive (B) Electronegative (C) Neutra...
With respect of chlorine, hydrogen will be:
(A) Electropositive
(B) Electronegative
(C) Neutral
(D) None of the above
Solution
Hint : To answer this question, we will first look at the modern periodic table and will understand the trends of electropositivity, electronegativity and size of the elements on moving down the group and on moving from left to right in a period. This is because all the discovered elements have been arranged according to their chemical properties and atomic numbers in the modern periodic table.
Complete Step By Step Answer:
We know that all the discovered elements have been arranged in the periods (horizontal rows) and groups (vertical columns) in the modern periodic table according to their atomic numbers and chemical properties.
Now, we will look at the modern periodic table:
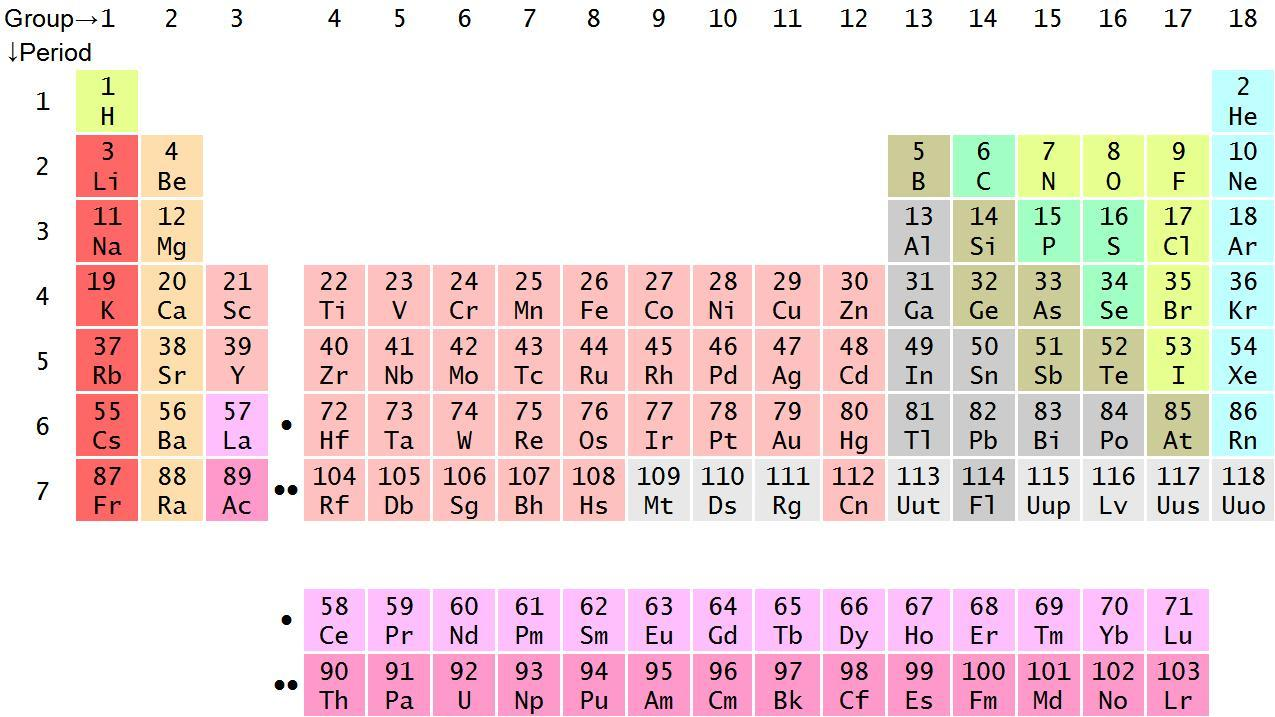
So, in the above periodic table, we can see that hydrogen is present in the extreme left and chlorine is present in the right side of the periodic table. We should now know some trends of periodic table such as:
On moving from left to right in a period, the atomic number and size of the element decreases and its electronegativity increases. So, we can conclude that the element (hydrogen) in the left side is electropositive in nature and the element (chlorine) in the right side is electronegative in nature.
Hence, with respect to chlorine, hydrogen will be electropositive.
Therefore, the correct option is (A) Electropositive.
Note :
We should note that electronegativity of an element also tells us about its ionization energy. If the element is highly electronegative, then it will not be able to lose its outermost electron easily and will have high ionization energy. If the element is electropositive in nature then it can easily lose its outermost electron and will have low ionization energy.
