Question
Question: Williamson's synthesis is used to prepare (A) Diethyl ether (B) PVC (C) Bakelite (D) Ethyl a...
Williamson's synthesis is used to prepare
(A) Diethyl ether
(B) PVC
(C) Bakelite
(D) Ethyl alcohol
Solution
The Williamson ether synthesis is an organic process in which an organohalide and a deprotonated alcohol are combined to create an Ether (alkoxide). Alexander Williamson invented this reaction in 1850. It usually includes a SN2 reaction between an alkoxide ion and a primary Alkyl halide. This reaction is significant in organic chemistry since it contributed to the discovery of the structure of ethers.
Complete answer:
The following is the general response mechanism: [Na]+[C2H5O]−+ C2H5Cl → C2H5OC2H5+ [Na]+[Cl]−
An SN2 bimolecular nucleophilic substitution mechanism is used in the Williamson ether synthesis. A backside assault of an electrophile by a nucleophile occurs in an SN2 reaction mechanism, and it occurs in a coordinated manner (happens all at once). A suitable leaving group that is highly electronegative, such as a halide, is required for the SN2 reaction to occur. An alkoxide ion ( RO− ) functions as the nucleophile in the Williamson ether reaction, attacking the electrophilic carbon with the leaving group, which is usually an alkyl tosylate or an alkyl halide. Because secondary and tertiary leaving sites prefer to continue as an elimination reaction, the leaving site must be a primary carbon. Due to steric hindrance, this reaction does not promote the synthesis of bulky ethers like di-tert butyl ether, and instead prefers the formation of alkenes.
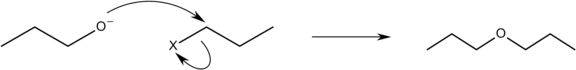
The Williamson reaction has a wide range of applications, is frequently employed in both laboratory and commercial synthesis, and is still the most straightforward and widely utilised way of producing ethers. It's simple to make both symmetrical and asymmetrical ethers. Epoxides are produced by the intramolecular interaction of halohydrins in particular. When it comes to unsymmetrical ethers, there are two options for reactant selection, and one is generally preferred based on availability or reactivity. The Williamson reaction is also commonly employed to make an ether from two alcohols indirectly. After converting one of the alcohols to a leaving group (typically tosylate), the two are reacted together.
Therefore, Williamson's synthesis is used to prepare Diethyl ether. So, option (A) is correct.
Note:
The Williamson reaction frequently competes with base-catalyzed alkylating agent elimination, and the type of the leaving group, as well as reaction circumstances (especially temperature and solvent), can have a significant impact on which is preferred. Some alkylating agent structures, in particular, can be particularly susceptible to removal. The Williamson reaction can compete with alkylation on the ring when the nucleophile is an aryloxide ion because the aryloxide is an ambident nucleophile.
