Question
Question: Why \({\text{S}}{{\text{c}}^{{\text{3 + }}}}\)salts are colourless whereas \({\text{C}}{{\text{r}}^{...
Why Sc3 + salts are colourless whereas Cr3 + salts are coloured?
Solution
Crystal field theory describes the removal of the degeneracy of d-orbitals of metal in presence of ligands. By the removal of degeneracy, the d-orbital of metal splits. The splitting explains many properties of transition metals like colours. The colour is produced by d-d transition. For d-d transition, unpaired electrons are required.
Complete step-by-step answer:
We will write the electronic configuration to determine the presence of electrons as follows:
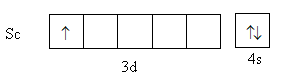
Scandium is a transition element with atomic number 21. Its valence electronic configuration is, 3d14s2 .
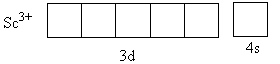
The charge on the metal is +3. The valence electronic configuration of Sc3 + is 3d04s0,
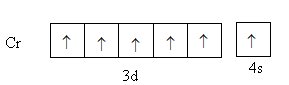
Chromium is a transition element with atomic number 24. Its valence electronic configuration is, 3d54s1 .
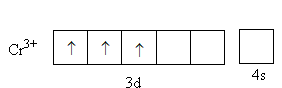
The charge on the metal is +3. The valence electronic configuration of Cr3 + is 3d34s0,
When ligands approach the metal, a complex forms. Metal’s d-orbitals split into two sets of two and three orbitals. In octahedral geometry of the complex, ligand approach from the axis and the orbitals dx2−y2 and dz2 are present on the axis whereasdxy,dzy and dxz orbitals lie in between the axes. So, the energy of two orbitals dx2−y2 and dz2 increases due to the ligands and the energy of three orbitals decreases. As a result, five degenerate-orbitals lose that degeneracy and split into two groups.
The CFT diagram of the Sc3 + and Cr3 + is as follows:
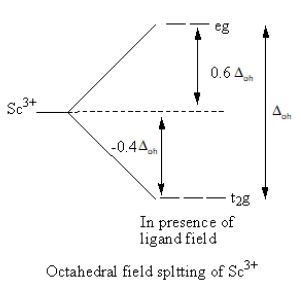
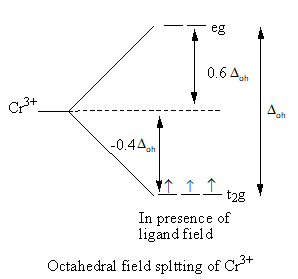
From the CFT diagram, we can say that the Sc3 + has no d-electron so, no d-d transition is possible so, the Sc3 + is colourless whereas the Cr3 + has three unpaired electrons so, the d-d transition is possible so, the Cr3 + is coloured.
Note: Colour of 3d−transition metals depend upon the d-d-transition and number of unpaired electrons in d-orbitals. Transmitted colour appears as colour of substance due to the presence of electrons that are responsible for transition. The presence of unpaired electrons also tells the magnetic nature of the complex. The given titanium complex is paramagnetic due to the presence of one unpaired electron. If all the electrons are paired then the complex will be diamagnetic.
