Question
Question: Why isn't allene planar?...
Why isn't allene planar?
Solution
Allenes are organic compounds in which one carbon atom forms double bonds with both of its carbon centres. Cumulated dienes are what allenes are categorised as. This class's parent chemical is propadiene, which is also known as allene. Cumulenes with X=C=Y bonding are a broader family of chemicals that have an allene-type structure but have more than three carbon atoms.
Complete answer:
Allenes have two sigma bonds and two pi bonds formed by the core carbon atom. The two terminal carbon atoms are sp2-hybridized, while the centre carbon atom is sp-hybridized. The bond angle created by the three carbon atoms is 180∘ , suggesting that the centre carbon atom has linear geometry. The two terminal carbon atoms are flat and twisted 90 degrees from one another. The structure can alternatively be thought of as a "extended tetrahedral" with a shape comparable to that of methane, a comparison that is carried over into the stereochemical study of several derivative compounds.
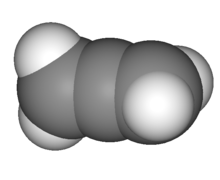
3D view of propadiene
Organic chemists have long been captivated by the symmetry and isomerism of allenes. There are two twofold axes of rotation through the central carbon atom, inclined at 45∘ to the CH2 planes at either end of the molecule, for allenes with four identical substituents. As a result, the molecule can be compared to a two-bladed propeller. A mirror plane goes across both CH2 planes, while a third twofold axis of rotation passes via the C=C=C bonds. As a result, this molecular type belongs to the D2d point group. An unsubstituted allene has no net dipole moment due to its symmetry.
Note:
Because there are no longer any mirror planes, an allene with two distinct substituents on each of the two carbon atoms will be chiral. Jacobus Henricus van 't Hoff predicted the chirality of these kinds of allenes in 1875, but it wasn't confirmed experimentally until 1935. The configuration of the axial chirality may be established by examining the substituents on the front atom followed by the rear atom when seen along the allene axis where A has a higher priority than B according to the Cahn–Ingold–Prelog priority criteria. Only the groups with the highest importance should be evaluated for the back atom.
