Question
Question: Why is vanillin achiral?...
Why is vanillin achiral?
Solution
A molecule is said to be achiral when it has a superimposable mirror image. To identify a molecule as achiral it should not have a chiral centre, i.e. no carbon atom should have 4 different groups attached to it. As they are achiral they do not have a centre or plane of symmetry.
Complete Step By Step Answer:
To find out if Vanillin is achiral we will look into its molecular structure.
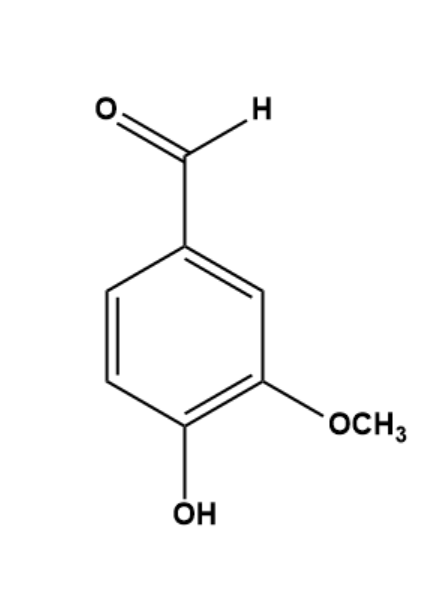
The structure of Vanillin shows the presence of an aldehyde group, an ether group, and a hydroxyl group. The IUPAC name of Vanillin is 4-hydroxy-3-methoxy benzaldehyde. This shows aldehyde is the group with the highest preference, followed by hydroxyl and then ether.
The Carbon atom attached to the Aldehyde group is attached to three other carbon atoms only. In the aromatic ring, no carbon atom is attached to 4 different groups forming a chiral centre except the methyl group in ether. Also, in the methyl group hydrogen is attached to 3 of the sides which does not make it chiral. And we established the fact that a chiral centre is important for a compound to be Chiral. Also, it has a mirror plane which means it is coplanar.
Therefore, Vanillin is achiral.
Note:
Superimposable mirror images are those which overlap each other when placed on top of each other. Always keep in mind non- superimposable images do not overlap and are the characteristic of enantiomers. Here Vanillin is a phenolic aldehyde since it contains a hydroxyl group at the para position.
